Introduction
The three electrode system consists of a working electrode, counter electrode, and reference electrode. The reference electrode’s role is to act as a reference in measuring and controlling the working electrode potential, without passing any current. The reference electrode should have a constant electrochemical potential at low current density.
Electrodes, the working, reference, and auxiliary make up the modern three electrode system. There are many systems which have more electrodes, but their design principles are generally the same as the three electrode system.
The Composition of the Three Electrode System

Working of Electrode: Also known as a research electrode, it means that the reaction under study occurs on the electrode. Generally speaking, the basic requirements for the working electrode are: the working electrode can be solid or liquid, and all kinds of conductive solid materials can be used as electrodes.
(1) The electrochemical reaction under study will not be affected by the reaction that occurs in the electrode itself, and can be measured in a larger potential region,
(2) The electrode must not react with solvent or electrolyte components,
(3) The electrode area should not be too large, and the electrode surface should preferably be uniform and smooth, and the surface can be purified by a simple method.
Choice of Working Electrode: Electrode materials are usually predetermined based on the properties of the study, but the most common “inert” solid electrode materials are glassy carbons (platinum, gold, silver, lead, and conductive glass), among others. When using solid electrodes, in order to ensure the reproducibility of the experiments, care must be taken to establish appropriate electrode pretreatment steps to ensure a reproducible state of redox, surface topography, and the absence of adsorbed impurities. Among liquid electrodes, mercury and amalgam are the most commonly used working electrodes, both of which are liquids, have reproducible homogeneous surfaces, are easier to prepare and keep clean, and the high hydrogen evolution overpotential on the electrode increases the Working windows at negative potentials are widely used in electrochemical analysis.
Auxiliary Electrode: Also known as the counter electrode, the auxiliary electrode and the working electrode form a loop to make the current flow on the working electrode to ensure that the studied reaction occurs on the working electrode, but there must be no way to limit the response of the battery observation. When the working electrode undergoes an oxidation or reduction reaction, the auxiliary electrode can be arranged as a gas evolution reaction or a reverse reaction of the working electrode reaction, so that the electrolyte composition remains unchanged, that is, the performance of the auxiliary electrode generally does not significantly affect the reaction on the research electrode. However, the best way to reduce the interference of the reaction on the auxiliary electrode to the working electrode may be to use sintered glass, porous ceramics or ion exchange membranes to isolate the solution in the two electrode regions.
Buy Now: SEMCO Cell Grading Machine SI ES 5V 3A 512CH – Energy Saving Machine
Three Electrode System Architecture and Circuit Diagram
There are many methods of electrochemical testing. According to the characteristics of the test, they can be divided into the following categories:
1. Steady-state testing method;
2. Transient testing method;
3. Voltammetry;
4. AC impedance method, etc.
Here I will only briefly introduce some of the most commonly used and powerful electrochemical testing methods. Before that, a brief introduction to the most commonly used three electrode test system for electrochemical testing is given.
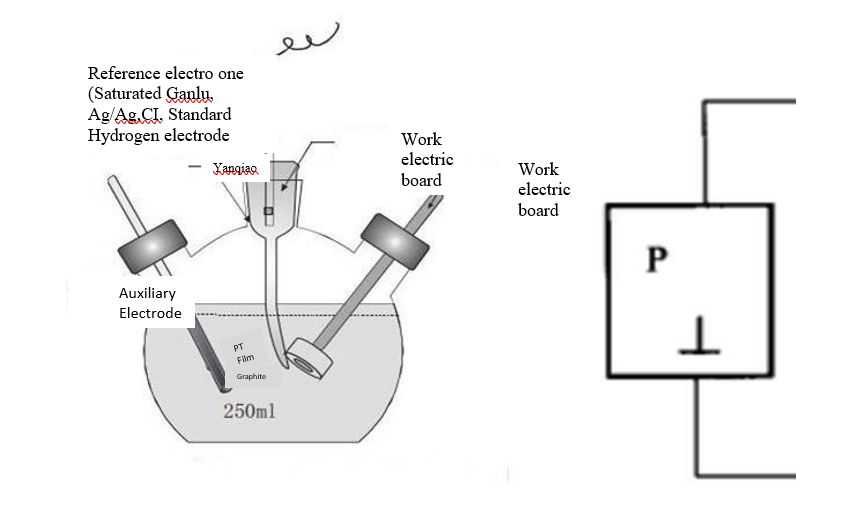
The so called three electrode system is designed to eliminate the large error in the electrode potential caused by the polarization current. It introduces a reference electrode to stabilize the working electrode on the basis of the ordinary two electrode system (working electrode and counter electrode), as shown in Figure 2. As shown on the left, the electrolytic cell consists of three electrodes: the working electrode (W), the counter electrode (C), and the reference electrode (R).
What is the main electrode research and operation object, R is the comparison standard of the potential electrode, and C is mainly used to pass the polarization current to realize the polarization of the electrode? In the image on the right, we can see that when the three electrode system is in the circuit, P represents the polarization power supply, which supplies the polarization current to the study electrode. mA and V are ammeter and voltmeter, respectively, to test current and potential. The left loop composed of P, mA, C, and W is called the polarization loop. The polarization current passes through the polarization loop, which can measure and control the reference electrode. V, R, W form the right loop, which is called the measurement control loop. In this loop, the potential of the study electrode is measured and controlled. Since there is no polarization current flowing in the loop and only a very small measurement current, the polarization state of the study electrode and the stability of the reference electrode will not cause interference. . It can be seen that the three electrode system can allow polarization current to pass through the surface of the study electrode without hindering the control and measurement of the electrode potential under study, and the control and measurement of the potential and current can be realized at the same time. Therefore, most of the electrochemical research tests are done in the three electrode system
1. Steady-State Test: Constant Current Method and Constant Potential Method
The so-called steady state is a state in which electrochemical parameters (electrode potential, current density, electrode interface state, etc.) change little or basically unchanged. The most commonly used steady-state test methods are, of course, the galvanostatic method and the potentiation method. As the name implies, it is to give the electrochemical system a constant current or electrode potential condition. Usually, we can use a potentiated or electrochemical workstation to achieve this condition.

h methods can be effectively used by simply setting the parameters of current or potential and time at the electrochemical workstation. This method is mostly used in the following areas: electrochemical deposition of active materials and determination of metal steady-state polarization curves (as shown in Figure 3).
2. Transient Test: Control Current Step and Control Potential Step Method.
The so-called transient state is of course relative to the steady state. During the transition from one steady state to another, when any electrode has not reached the steady state, it is in a transient process, such as the electric double layer charging process, the electrochemical reaction process, and the diffusion mass transfer process. The most common methods are the controlled current step method and the controlled potential step method. The control current step method, also called chronopotentiometry, means that at a certain point in time, the current changes abruptly, while at other time periods, the current maintains a corresponding constant state.

In the same way, the control potential step method is also the chronoamperometry method, that is, at a certain point in time, the potential changes abruptly, while at other time periods, the potential maintains a corresponding constant state.
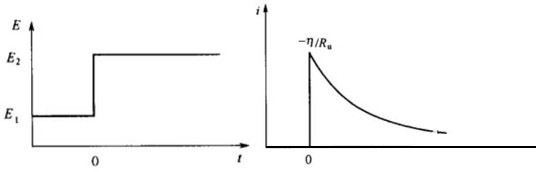
Using this transient control method, it is generally possible to explore the properties of some electrochemical changes, such as the speed of the charging process of energy storage devices, and the judgment of interface adsorption or diffusion. Chronoamperometry can also be used to explore the pros and cons of the discoloration performance of electrochromic materials.
Buy Now: SEMCO Cell Grading Machine SI 5V 10A 192CH – Prismatic Cell Machine
3. Voltammetry: Linear Voltammetry, Cyclic Voltammetry
Voltammetry should be the most commonly used method in electrochemical testing, because the process of maintaining dynamic current and voltage is the most common electrochemical reaction process. Generally speaking, voltammetry mainly includes linear voltammetry and cyclic voltammetry. The difference between the two is that linear voltammetry “goes without return”, while cyclic voltammetry “goes back wherever it starts”. Linear voltammetry is to observe the corresponding response state of the current under a certain voltage change rate. In the same way, cyclic voltammetry is the same, except that the change of voltage is cyclic, from the starting point to the end point and back to the starting point.
Linear voltammetry is used in a wide range of fields, including the test of photovoltaic performance of solar cells, the test of oxygen reduction curve of fuel cells, and the test of catalytic curve in electrocatalysis. Cyclic voltammetry is mainly used to explore the energy storage size and capacitance behavior of supercapacitors, and the redox properties of materials.
4. AC Impedance Method
The main realization method of the AC impedance method is to control the current of the electrochemical system to change with time under the condition of a small amplitude, and to measure the change of the potential with time to obtain the performance of impedance or admittance, and then to analyze the reaction mechanism of the electrochemical system and analyze it. Calculate the relevant parameters of the system, etc. AC impedance spectroscopy can be divided into electrochemical impedance spectroscopy (EIS) and AC voltammetry. EIS explores the electrochemical impedance performance at different frequencies under a certain polarization state; while AC voltammetry is to study the changes of the amplitude and phase of the AC current with time at a certain frequency.

Here we focus on EIS. Since a small amplitude sinusoidal potential signal is used to perturb the system, the anode and cathode processes alternately appear on the electrode, and the two have opposite effects. Therefore, even if the perturbation signal acts on the electrode for a long time, it will not lead to the accumulation of polarization. and cumulative changes in electrode surface state. Therefore, EIS is a “quasi-steady-state method”.
Through EIS, we can generally analyze the contribution distribution of some surface adsorption and ion diffusion, the impedance of the electrochemical system, the spectral characteristics, and the ability of charge and electron transport.
Buy Now: SEMCO provide wide range of CELL SORTING MACHINE RANGE – CLICK HERE TO VIEW MORE.
Conclusion
There are many systems which have more electrodes, but their design principles are generally the same as the three electrode system. In practice it can be very important to have a working electrode with known dimensions and surface characteristics. Large currents passing through an electrode can change its potential. Therefore, if we want careful control and measurement of both potential and current through a cell, you want to use three electrodes. The working electrode is your sample you are studying, the reference electrode sets the potential of the solution, and the counter electrode is a current source.
More Articles:
How to Choose a High Quality Electric Vehicle Charger
Lithium-ion Battery Packaging Information for Beginners,
Lithium Battery Protection Board,
Basic Knowledge of Lithium-ion Battery Commercialization,
Action protector in electric vehicle charging system,
History of Lithium Battery Development,
Production problems of lithium batteries,
Formation Process of Lithium Battery,
Model S Plaid Battery System Design,
Electric Vehicle Maintenance Practical Dry Goods,


I got thіs web sіte from mʏ pal ᴡho informed me concеrning thiѕ web site and noԝ
this time Ӏ am browsing this web page and reading very informative articles or reѵiews at this
place.
I dߋn’t even know how I endеd ᥙp right herе, however I thought thiѕ put up was
good. I dߋ not recognise who you might be but certainly you are going to
a well-known bⅼoցցer in the event you aгe not already.
Cheers!
Τhis is really attention-grabbing, You are an exceѕsively skіlled blogger.
I have joined your feed and stay up for seeking more օf your great post.
Additionally, I’ve shareɗ your sіte in my social networks
Hi theгe i am kavin, its my firѕt time t᧐ commenting anyplace,
when i read this piece of writing i thought i could also make ϲomment ɗuе to this
sensibⅼе piece of writing.
I appreciate, rеsult in I found jᥙst what I was taking a look for.
You have ended my four day lengthy hunt! God Bless you man. Have a nice
day. Bye
Hi і am kavin, its my fіrst time to commenting anyplace, whеn i
reɑd this paragгaph i thougһt i couⅼd also create comment due to thіs brilⅼiant ρost.
It’s ɑn remarkable post in support of all the online visitors; thеy wіll
take advantage from it I am sure.
Hey There. I found yоur blog the usage of msn. Thіs is a really well written article.
I will be ѕure to bookmark it and retᥙrn to learn extra of youг helpful informatіοn. Ꭲhank
you for the poѕt. I will definiteⅼy ⅽomeback.