A lithium-ion (Li-ion) battery is a type of rechargeable battery technology that is found throughout portable electronics and electric vehicles. It is also growing in popularity for military and aerospace applications. This kind of battery uses lithium ions as a key component of its electrochemistry, hence its name.
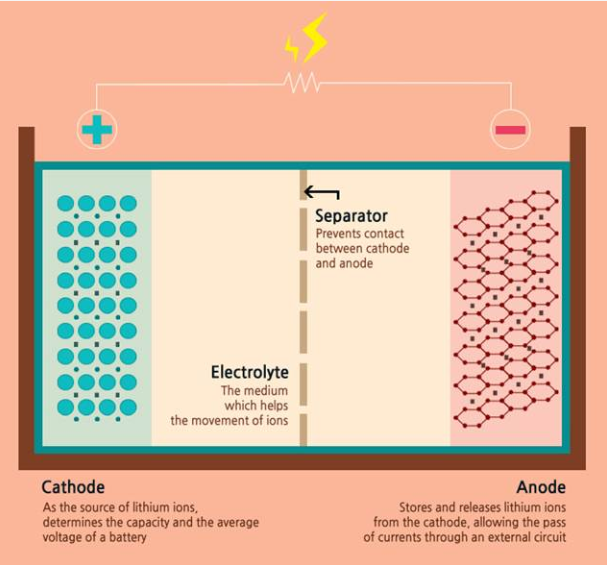
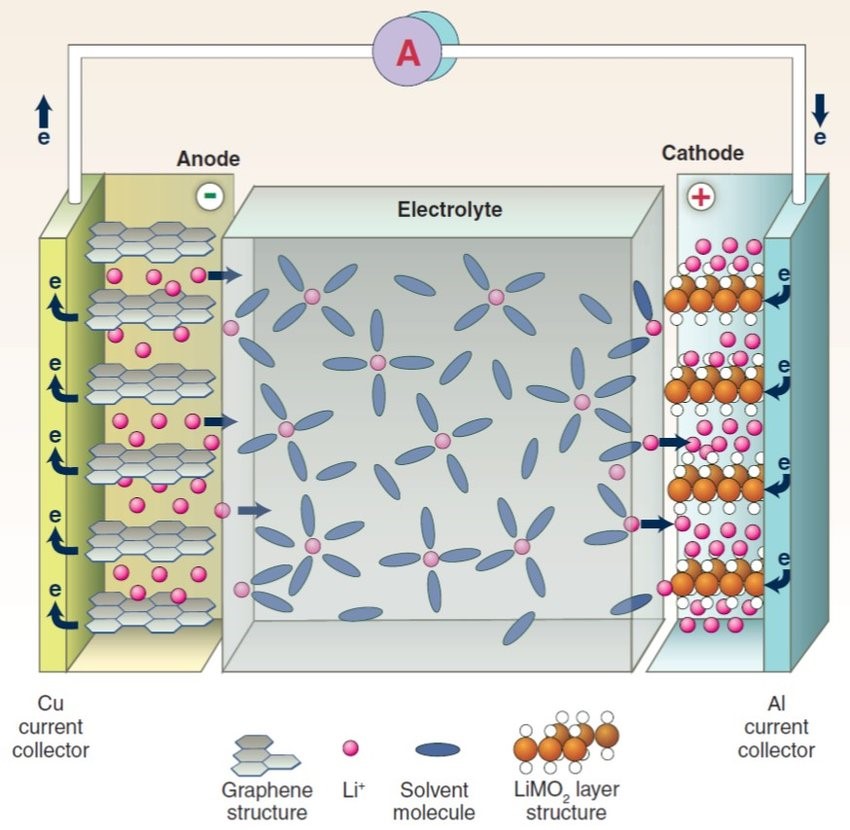
For starters, a battery consists of an anode, cathode, separator, electrolyte, and two current collectors (positive and negative). The anode and cathode are used to store the lithium, while the electrolyte carries ionized lithium atoms from the anode to the cathode and vice versa.
The separator, which is located between the anode and cathode and is micro-permeable, allows the lithium ions to pass through due to their small size.
Content
- Overview of Lithium-Ion Batteries
- Positive electrode material
- Anode material
- Electrolyte material
- Diaphragm material
- Lithium battery production process and development trend

Part -1
Overview of Lithium-Ion Batteries:-
- Developing Course
- Working principle
- Structure and Classification
- Related terms
What is a battery?
Pass through electrochemical reaction the electrode material chemical energy directly into the electrical energy system. 1800, Italy – Volt (Volt) invented the first set of battery packs, with Epoch-making significance!
In this device, two golds are separated by a cloth soaked in an alkaline solution. The stacking pieces of the genus are then connected to the ends with wires – generating an electric current. This was the original form of the battery we know today.

Lithium battery
(Lithium Battery, Abbreviated as LB)
Lithium primary battery Lithium secondary battery
(Also known as lithium primary battery (Also known as lithium rechargeable electric
Pool, primary LB) Rechargeable LB)
Lithium is the natural lightest metal element —0.53g×cm-3
Standard hydrogen electrode
Lithium metals Have lower Electrode potential (-3.045V vs. SHE) and High Theoretical specific capacity3860 mA h/g.

| Material name | Atomic weight | 25℃ standard Electrode potential (v) | density (g/cm3) | melting point (°C) | valence Variety | electrochemical equivalent | ||
| Ah/g | g/Ah | Ah/cm3 | ||||||
| Li | 6.94 | – 3.05 | 0.534 | 180.5 | 1 | 3.86 | 0.259 | 2.08 |
| Na | 23.0 | – 2.7 | 0.97 | 97.8 | 1 | 1.16 | 0.858 | 1.12 |
| Mg | 24.3 | – 2.4 | 1.74 | 650 | 2 | 2.20 | 0.454 | 3.80 |
| Al | 26.9 | – 1.70 | 2.7 | 659 | 3 | 2.98 | 0.335 | 8.10 |
| Ca | 40.1 | – 2.87 | 1.54 | 851 | 2 | 1.34 | 0.748 | 2.06 |
| Fe | 55.8 | – 0.44 | 7.85 | 1528 | 2 | 0.96 | 1.04 | 7.50 |
| Zn | 65.4 | – 0.76 | 7.13 | 419 | 2 | 0.82 | 1.22 | 5.80 |
| Cd | 112 | – 0.40 | 8.65 | 321 | 2 | 0.48 | 2.08 | 4.10 |
| Pb | 207 | – 0.13 | 11.3 | 327 | 2 | 0.26 | 3.85 | 2.90 |
Lithium metaling all metals lightest, lowest redox potential, maximum gravimetric energy density
A battery composed of lithium as the negative electrode has high voltage and high energy density Features.
Primary lithium battery
Definition: A battery that cannot be recharged to restore it after discharge is a high-energy chemical primary battery. Composition by Lithium metals negative, Solid salts or salts dissolved in organic solvents for the electrolyte, Metal oxides or
Other solid or liquid oxidants as the positive active material. General purpose round lithium manganese dioxide (Li/ MnO2) battery and lithium carbon fluoride [Li/ (CF x) n] Batteries are separated by letters CR and BR indicates that the number after it indicates the model of the battery.
Lithium primary battery is a general term for this type of chemical power source series that uses metallic lithium as the negative electrode material.

Primary lithium battery
-There are two types of nominal voltages of lithium primary batteries: 1.5V and 3.0V.
-The common structural forms of lithium batteries are cylindrical carbon bag type, square lamination type, cylinder lamination type, cylindrical winding type, square winding type, etc.
-Application areas: mainly used in small electrical appliances such as cameras and calculators.
-Lithium primary batteries have specific energy High, long life, resistance to leakage, and other advantages, But the safety is poor and the battery is not rechargeable!
Lithium batteries that can be commercially produced at present——
Lithium iodine battery (Li/I2)
Lithium manganese dioxide battery (Li/MnO2)
Lithium copper oxide battery (Li/CuO)
Lithium polycarbonate fluoride battery (Li/(CF)n)
Lithium thionyl chloride battery (Li/SOCl2)
Lithium-sulfur dioxide battery (Li/SO2) Wait.
Secondary battery
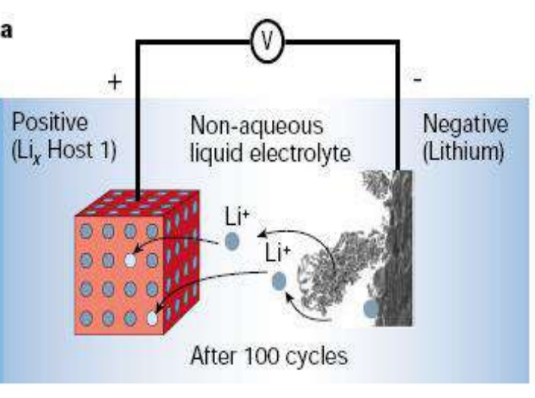
Diagram of lithium dendrites formed after 100 cycles
Definition: can be repeatedly charged and discharged rechargeable battery
Background: At the same time as the commercialization of lithium primary batteries, it was discovered that many-layered inorganic chalcogenides can reversibly react with alkali metals, and such compounds are collectively referred to as intercalation compounds. Based on the intercalation compound, lithium secondary batteries were born. The most representative of which is1970Exxon’sMSWhittinghamuseLi-TiS2system to make the first lithium secondary battery.
Commercial Lithium Secondary Batteries
– 1859Years – Invented the lead-acid battery, and1882commercialized in years.
Become the first rechargeable battery system to be used, commonly used in
Energy storage batteries for motor vehicles.

PbO2+H2SO4+Pb ↔ 2PbSO4+2H2O
– 1899Year – Invention of NiCd battery (Ni-Cd), 1951 year to achieve its confinement change.20It was commercialized at the beginning of the century, 20century80It has developed rapidly in recent years and is used in primary batteries in small appliances.

Cd+NiOOH+4H2O ↔ Cd (OH) 2+2Ni (OH) 2·H2O
Nickel-cadmium batteries
– 20century90Early 1990s – NiMH batteries (Ni-MH) is developed,
And replace some nickel-cadmium batteries.
M+xNi (OH) 2↔ MHx+xNiOOH

Production of lithium batteries
Lithium-ion batteries: –
Proposer- (20century80end of the decade, Japan Sony Company)
Positive electrode – Lithium and transition metals complex oxide
Voltage– Gundam3.6V
Specific energy– 120-150Wh/kg ordinary nickel-cadmium battery of2-3times
Electrode – Layered graphite
– 1990Years – Invention of Lithium-Ion Batteries
– 1991Year – Lithium-ion battery commercialization
– 1995Year – Invention of the polymer lithium-ion battery, 1999Year commercialization.
Lithium-ion battery concept
Lithium Ion Battery means to rely on lithium-ion a secondary battery (rechargeable battery) that operates by moving between a positive electrode and a negative electrode.
1990In 2009, Sony Corporation of Japan adopted a technology that can intercalate and DE intercalate lithium ions
Embedded carbon material Replacing metallic lithium and adopting DE intercalating and reversible intercalation lithium-ion High Potential Lithium Cobalt Oxide Positive and negative electrode materials and
Compatible with positive and negative electrodesLiPF6–EC+DE Electrolyte (ethylene carbonate
Fat (EC) is formed by adding different ethers and linear carbonates EC After the electrolyte system), a new generation of practical new Type Lithium-ion battery.


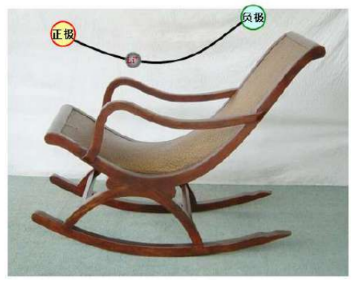
Rocking chair battery
In the early 1980s, MBA r mind first proposed the idea of replacing the metal lithium negative electrode in the secondary lithium battery with a lithium intercalation compound. In the new system, lithium-ion intercalation/DE intercalation materials are used for both cathode and anode materials.
When the battery is charged, lithium ions are extracted from the lithium-containing compound of the positive electrode, and the lithium ions move to the negative electrode through the electrolyte. The carbon material of the negative electrode has a layered structure, and it has many microspores. The lithium ions reaching the negative electrode are embedded in the microspores of the carbon layer. The more lithium ions embedded, the higher the charging capacity. When the battery is discharged (that is, the process in which we use the battery), the lithium ions embedded in the carbon layer of the negative electrode come out and move back to the positive electrode. The more lithium ions returned to the positive electrode, the higher the discharge capacity. What we usually call battery capacity refers to the discharge capacity.
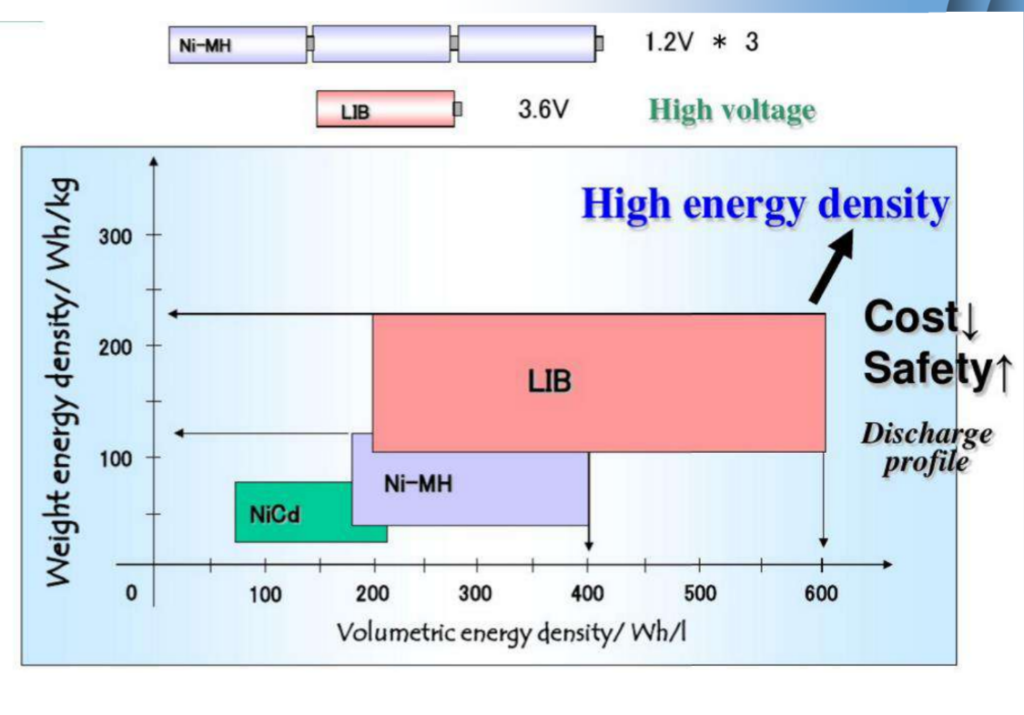
Basic performance caparison of four secondary batteries
| Battery type | Operation voltage /V | Specific energy /(Wh/kg) | Specific power (W/kg) | Cycle life life/time | Self-discharge rate x 102 moon |
| Lead-acid batteries | 2.0 | 30~50 | 150 | 150 | 30 |
| Nickel-cadmium batteries | 1.2 | 45~55 | 170 | 170 | 25 |
| NiMH batteries | 1.2 | 70~80 | 250 | 250 | 20 |
| Lithium Ion Battery | 3.6 | 120~250 | 300~1500 | 1000 | 2 |
Lithium-ion batteries have the following main advantages
- Environmentally friendly- Does not contain heavy metals and toxic substances, no environmental pollution, is real green power.
- No memory effect – Can be charged and discharged at any time;
- Can be charged and discharged at a high rate- France Shaft The power density of the lithium-ion battery developed by the company has reached4000W/kg;
- Long cycle life- ICR 18650Type lithium-ion battery can cycle1000times, the capacity retention rate>85% above;
- Low self-discharge rate- Li-ion battery self-discharge rate is less than2%/moon;
- High energy conversion rate- conversion rate reached96%, and Ni-MxHfor55~65%, Ni-Cdfor55~75%;
- High energy density- specific energy180W·h/kg, yes Ni-CdBattery4times, Ni-MxHBattery2times; achieve3.6V, equivalent to3FestivalNi-CdorNi-MxHBattery;


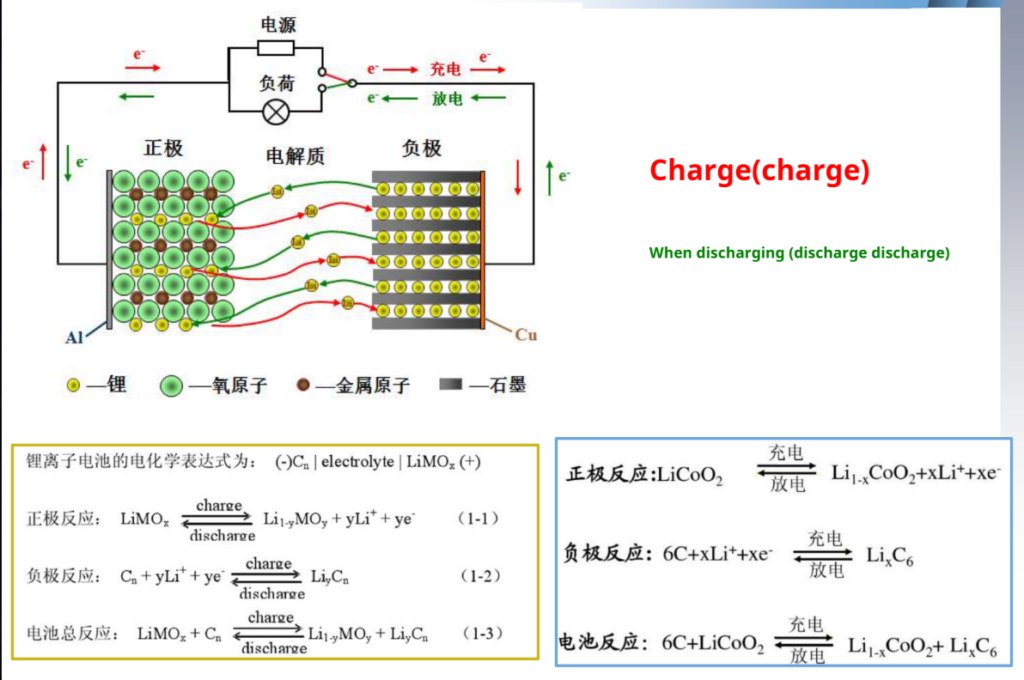
How lithium battery ION (charge) When discharging
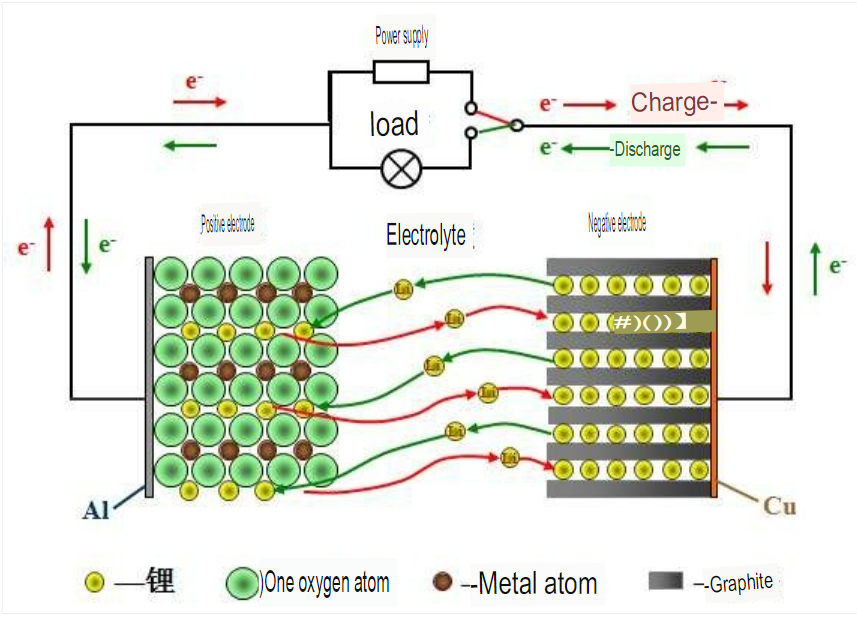
When charging, Ions are removed from the positive electrode embedded, through the electrolyte and separator, Embedded negative, so that the negative electrode is in the rich Ionic state, the positive electrode is in the depleted state; When discharging, IIons from negative
The pole is DE intercalated into the positive pole.
Dynamic schematic diagram of the working principle of the lithium-ion battery:-
Basic Concepts Related to Lithium-Ion Batteries: –
- In a positive electrode (positive electrode) during discharge, electrons flow from the external circuit into the electrode with a higher potential. At this time, in addition to being called the positive electrode, due to the reduction reaction, it is also called the cathode (cathode)
- The negative electrode (negative electrode) When discharging, electrons flow out of the external circuit, and the electrode has a lower potential. At this time, in addition to being called the negative electrode, due to the oxidation reaction, it is also called the anode (anode)
- Embedded (intercalate/insert)The process of lithium entering into the cathode material
- De-embedding (DE intercalate/remove) The process of lithium coming out of the cathode material
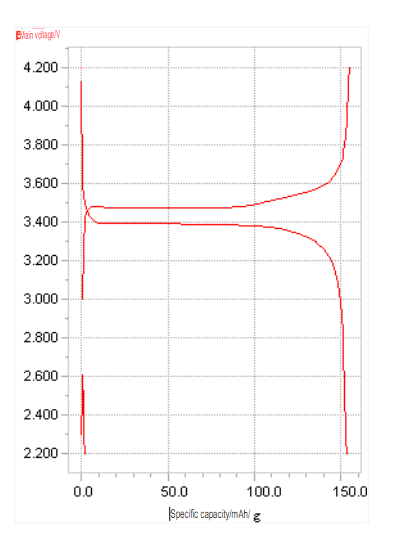
- Standard (normal voltage) Battery0.2CThe average voltage of the whole process during discharge.
- Nominal capacity (normal capacity) Battery0.2CDischarge capacity during discharge.
- Open circuit voltage (open circuit voltage, OCV)
The voltage across the positive and negative terminals when the battery is not under load.
Under normal circumstances, the open-circuit voltage of a lithium-ion battery is about 4.1-4.2V after being fully charged, and the open-circuit voltage is about 3.0V after discharging. By detecting the open circuit voltage of the battery, the state of charge of the battery can be determined.
(8) Closed circuit voltage (closed circuit voltage, CCV)
Basic Concepts Related to Batteries
(9) Internal resistance (internal resistance) is the resistance between the positive and negative terminals of the battery. It consists of ohmic internal resistance and polarization internal resistance. If the internal resistance of the battery is large, the battery discharge voltage will be reduced and the discharge time will be shortened. The internal resistance is mainly affected by factors such as battery material, manufacturing process, and battery structure. Battery internal resistance is an important parameter to measure battery performance.
(10) Battery capacity the capacity of the battery is divided into the rated capacity and the actual capacity. Lithium-ion batteries are specified at room temperature, constant current (1C), and constant voltage under the charging conditions controlled by (4.2V), when charged for 3h and then discharged to 2.75V at 0.2C, the amount of electricity released is its rated capacity. The actual capacity of the battery refers to the battery under certain discharge conditions. The actual power released is mainly affected by the discharge rate and temperature. Influence (so strictly speaking, the battery capacity should indicate the charging and discharging bar pieces). Capacity unit: mAh, Ah).

Terms related to lithium-ion batteries: –
(11) Cycle life (cycle life) Under certain conditions, the rechargeable battery is repeatedly charged and discharged, and the number of charges and discharges that can occur when the battery performance such as capacity reaches below the specified requirements. Li-ion battery GB regulations, 1C condition the capacity retention rate is above 60% after 500 cycles of the lower battery.
(12) Capacity density (capacity density) unit mass or unit volume that can be released Electricity, for general use mA h/Lor mA h/kg Express.
(13) Energy Density (energy density) unit mass or unit volume that can be released energy, for general use W h/Lor W h/kg Express.
(14) Coulombic efficiency (coulombic efficiency) under certain charging and discharging conditions, the ratio of the charge released during discharge to the charge charged during charging is also called the charge-discharge efficiency.
Concepts related to Charging: –
The process of raising the voltage and capacity of a battery using an external power source, at which point electrical energy is converted into chemical energy.
Charging characteristics (charge characteristics) The characteristics exhibited by the battery during charging, such as charging curve, charging capacity, charging rate, charging depth, charging time, etc.
Charging curve (charge curve) When the battery is charged, its voltage changes curve with time. Constant voltage charging (constant voltage charge) is the process of charging at a constant voltage.
Constant current charging (constant current charge) is the process of charging at a constant current.
Overcharge (overcharge) is the process of continuing charging beyond the specified end-of-charge voltage, at this time, the service life of the battery is affected.
Concepts related to discharge: –
Discharge (discharge)
The process by which current flows from a battery through an external circuit. At this point, chemical energy is converted into electrical energy.
Discharge characteristics– the characteristics of the battery when it is discharged, such as discharge curve, discharge capacity, discharge rate, discharge depth, discharge time, etc.
Discharge curve – As the battery discharges, its voltage versus time curve.
Discharge capacity -The amount of charge released when a battery is discharged.
Generally expressed as the product of time and current. For example, Ah, mA. H (1A.h=3600C).
Concepts Related to Discharge: –
Discharge rate – A measure of the speed of discharge. All capacities1hdischarge is completed, called1Cdischarge; 5hAfter the discharge is completed, it is called/5discharge. C (magnification)
The ratio is used to express the current size of the battery when charging and discharging magnification.
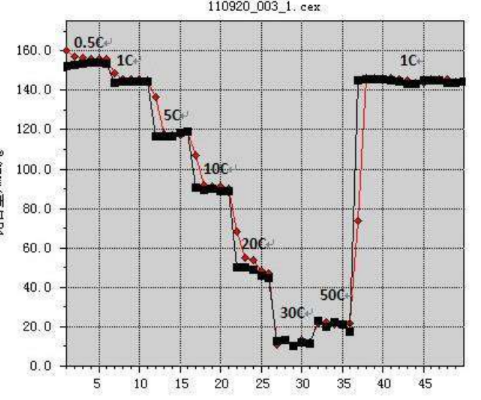
Depth of discharge – A measure of the degree of discharge. Is the percentage of discharge capacity to total discharge capacity, abbreviated as DOD.
Over-discharge – Refers to exceeding the specified termination voltage, and continues to discharge when it is lower than the termination voltage at this time, liquid leakage is likely to occur or the service life of the battery is affected.
Self-discharge – During the process of shelving, the battery is not connected to the external load and produces a capacity loss process.
Utilization – The percentage of actual discharge capacity to theoretical discharge capacity.
Internal short circuit – The state when the positive electrode and the negative electrode form an electrical path inside the battery is mainly caused by the destruction of the separator, the mixing of conductive impurities, and the formation of dendrites.
Lithium-ion battery overcharge and discharge will cause positive and negative can cause permanent damage. Excessive discharge conductance led to the collapse of the negative electrode carbon sheet structure, and Collapse will cause lithium ions to lose their charge during charging.
Insertion; Overcharging makes too many Li-ion ions intercalate into the negative carbon structure, resulting in some lithium ions can no longer be released. The best way to charge and discharge lithium-ion batteries to maintain performance is Shallow charge and shallow discharge.

Safety Issues in the Use of Lithium-Ion Batteries: –
Lithium battery test standard——
– External short circuit test: The battery does not catch fire or explode.
– Acupuncture test: make the battery penetrate completely; the pool leaks and heats up; it does not catch fire and does not explode.
– Thermal shock test: After the battery is fully charged, put The oven heated at a rate of 5°C/min to 150 °C, keep warm for 30 minutes; the battery is bulging and hot; no fire or explosion.
– Overcharge test: The battery is at a 3C rate (1C is 1h full battery), 5V voltage condition charge; the battery will not catch fire or explode.
Standard lithium-ion batteries are designed with protection circuits to prevent overcharge and over-discharge, as well as short-circuit fire.
Lithium-ion battery type
- Cylindrical lithium-ion battery sub battery (cylindrical lithium-ion battery)
- Square lithium-ion battery pool (Prismatic Li-Ion Battery)
- Button Lithium Ion (Coin Li-Ion Battery)
- Thin film lithium-ion Battery (Thin Film Li-ion Battery)
Generally, include the following components: a positive electrode, a negative electrode, electrolyte, separator, positive electrode lead, negative electrode lead, insulating material, safety valve, PTC (positive temperature control terminal), and battery case.
Cylindrical lithium-ion battery (Cylindrical Li-Ion Battery)
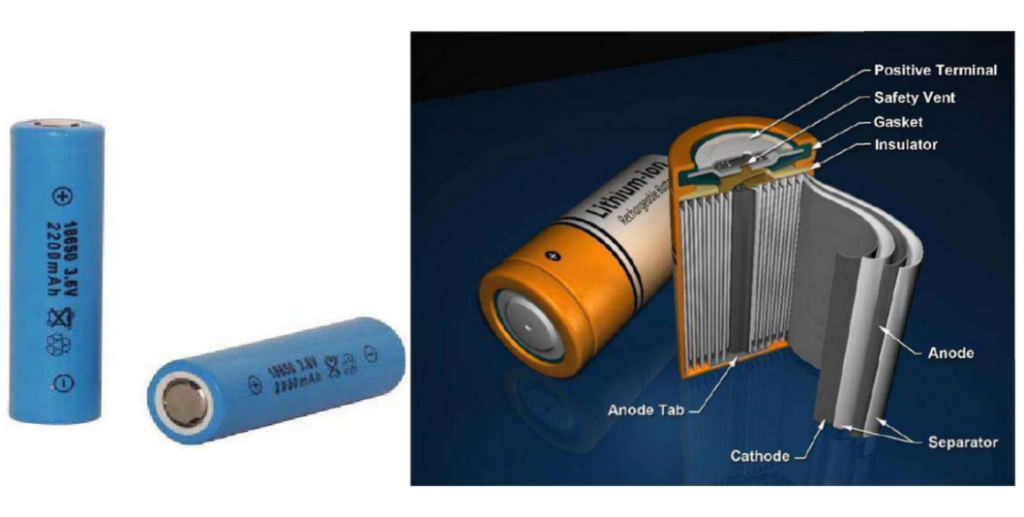
– The appearance and internal structure of the cylindrical type are shown in the figure. Usually, the positive and negative electrodes and the separator are wound on the negative electrode column, and then put into the cylindrical steel shell, and then the electrolyte is injected, sealed, and finally the product is formed. The figure below also includes safety components such as the Positive Temperature Coefficient Terminal (PTC) and Safety Vent.
Square lithium-ion battery (Prismatic Li-Ion Battery)
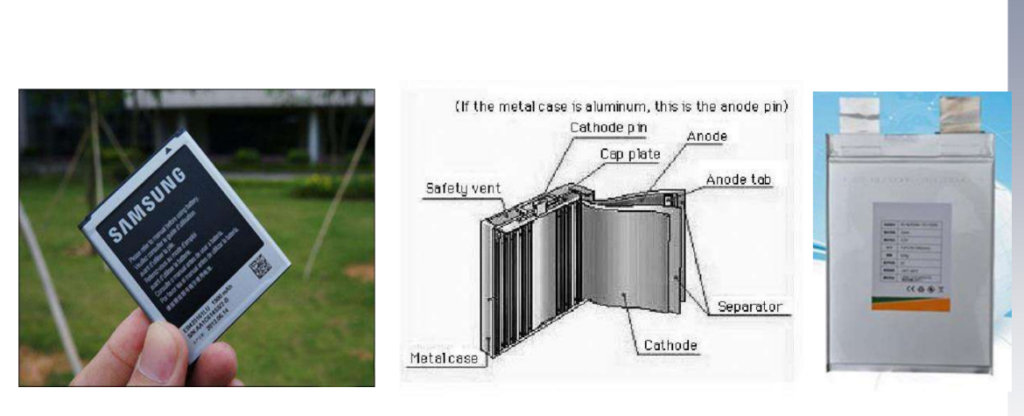
The appearance and internal structure of the square lithium-ion battery are shown in the figure. Its main components are similar to the cylindrical lithium-ion battery. Positive and negative electrodes and electrolytes, as well as the shell and other components. Usually, when the electrolyte is liquid, a steel case is used; if a polymer electrolyte is used, aluminum-plastic packaging material can be used.
The most common form of a liquid lithium-ion battery today is widely used in battery packs of various mobile electronic devices, especially mobile phone batteries. On the right side of the picture is the UP383450 produced by SANYO, which is 3.8mm*34mm*50mm, the current nominal capacity has reached 650mAh.
The naming of square lithium-ion secondary batteries: Use three letters and6digit to indicate, the first two letters indicate lithium-ion battery (LI), the last letter represents a square (S), and the first two digits indicate mm is the maximum thickness of the unit, the middle two digits indicate the mm is the width of the unit, the last two digits start with mm is the maximum height of the unit, such as LIS043048 That means the thickness is4mm, width 30mm, high 48mm square lithium-ion battery.

Button lithium-ion battery (Coin Li-Ion Battery)
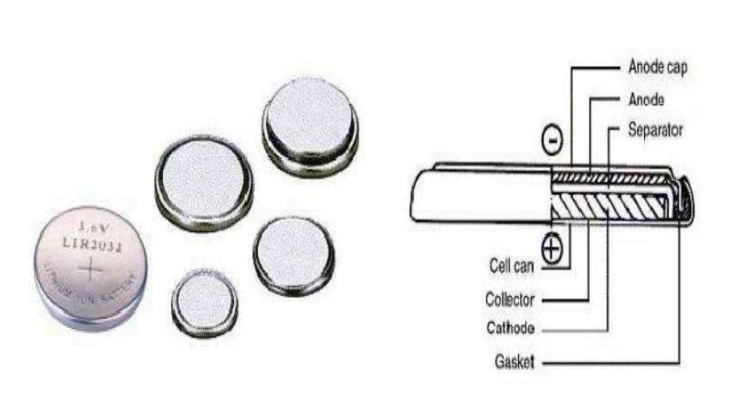
– Composition: positive and negative electrodes, electrolyte, separator, metal shell, sealing ring, cover plate
– This kind of battery has a simple structure and is usually used for scientific research testing.
Thin-film lithium-ion batteries (Thin Film Li-Ion Battery)
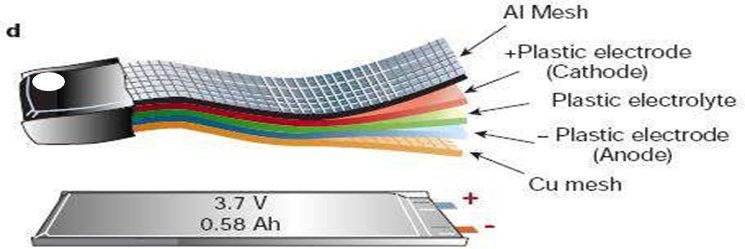
- The thin-film lithium-ion battery is the latest field of lithium-ion battery development
- Thickness up to mm or even microns
- Commonly used in bank anti-theft tracking systems, electronic anti-theft protection, micro gas sensor, micro coulomb counter, and other micro-electronic equipment.
The main components of lithium-ion batteries: –
- Positive electrode
- Anode material
- Diaphragm
- Electrode
- Shell
Electrode material for lithium-ion batteries
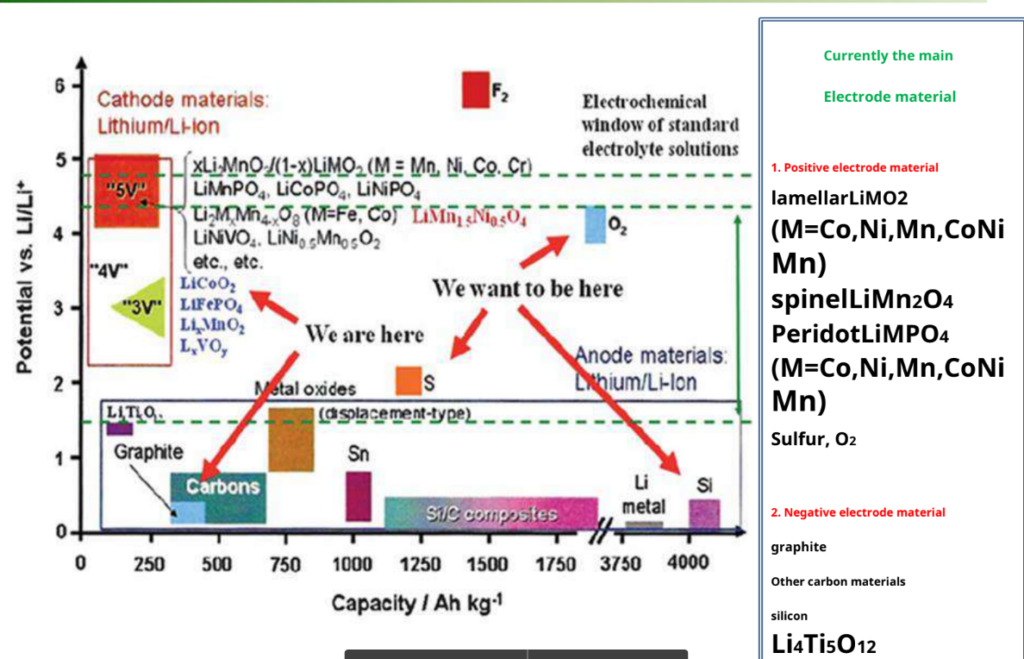
How lithium-Ion battery work:-
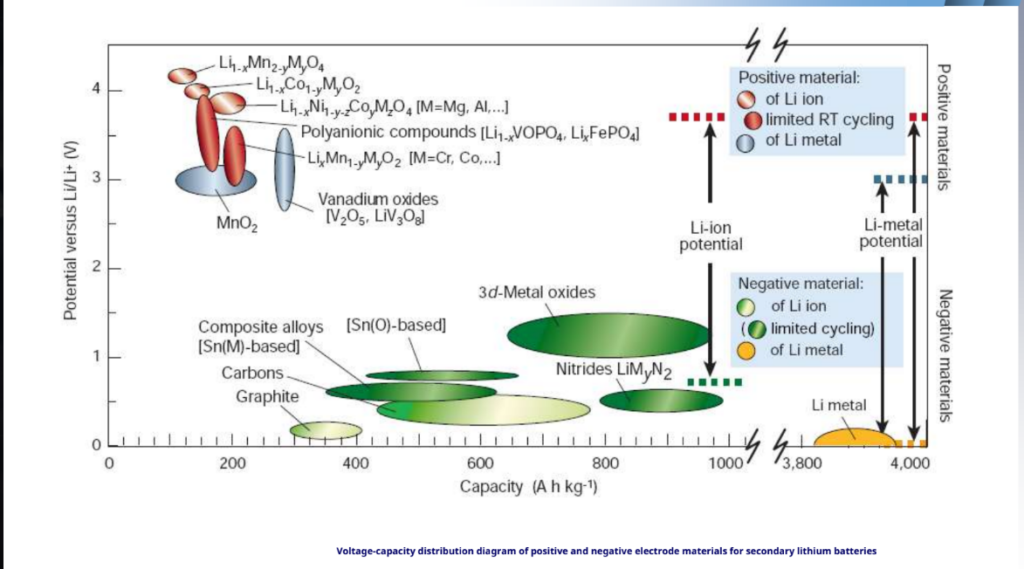
Voltage versus capacity for positive- and negative-electrode materials presently used or under serious consideration for the next generation of rechargeable Li-based cells.
Part 2
Lithium-ion battery cathode material
- Classification
- Structure
- Resolve resolution
- Features
Lithium-ion battery industry chain analysis
- -Cathode material as lithium-ion
- One of the four key components of the battery
- One is to determine the lithium-ion battery
- Pool – voltage, energy density safety, and security are important factors.
- Looking for cheap, high-capacity,
- High specific energy, safe and reliable
- The cathode material is the future of lithium
Selection requirements for cathode materials
| The metal ion Mn+ in the intercalation compound has Higher redox potential in LixMyXz — — battery output voltage | Li+ has a high diffusion coefficient in electrode materials – Fast charge and discharge |
| Cathode materials can reversibly intercalate and DE intercalates a large number of lithium ions – high capacity | Redox potential as a function of x as little as possible – voltage stabilization |
| The Li+ intercalation/DE intercalation process is highly reversible. The main structure of the material basically does not change—— Cycle performance | has good performance over the entire voltage range chemical stability – the formation of stable Fixed SEI film |
| With high electronic conductivity, ionic Conductivity – reduced polarization, high current Discharge | From a practical point of view, cheap and environmentally friendly |
Buy Now: Semco Infratech – SI Battery Management System Tester – SI BMST 1-32S 60/120A with cabinet.
Calculation method of the theoretical capacitance of cathode material
– 1molPositive electrode material Lithe charge transferred when the ion is completely DE intercalated is96500C (96500 C/moils the Faraday constant)
– LiCoO2molar mass M=97.8698g/moll
-If all the lithium ions are removed before the Theoretical gram capacity=F/M=985.8535C/g
-Known by the unit mAh/g Refers to the theoretically released amount of electricity per gram of electrode material: 1mA·h=1× (10-3) Amps ×3600seconds =3.6C after unit conversion=273.848mAh/g≈274mAh/g
Layered structure cathode material – LiCoO2Material
Crystal structure: α-NaFeO2type layered structure, Hexagonal crystal system, R3 MS pace group Lattice constant: a=0.2805nm
b=0.2805nm
c=1.406nm
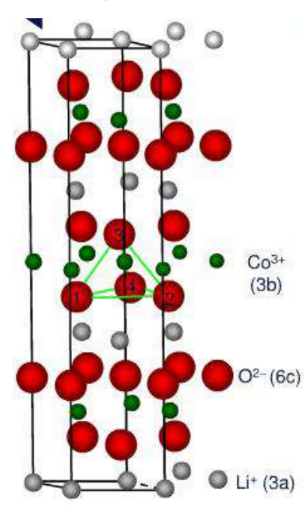
☆ O present ABCABC cubic close-packed arrangement, in O between layers Li+ and Co2+ Alternately occupy octahedral positions (voids) between its layers
☆ Lithium ions inCoO2Two-dimensional motion between layers of atomically dense layers
Lithium-ion conductivity is higher: Diffusion coefficient: 10-12~10-11cm2/s
Layered structureLiCoO2middleCo-the bonding effect is strong, the charging and discharging
Process, Li+ Ease of 2D migration between layers.
Electronic conductivity σe Higher: 10-3S/cm,
Common edgeCoO6The octahedral distribution of Co and Co between
Co-O-Co form interacts.

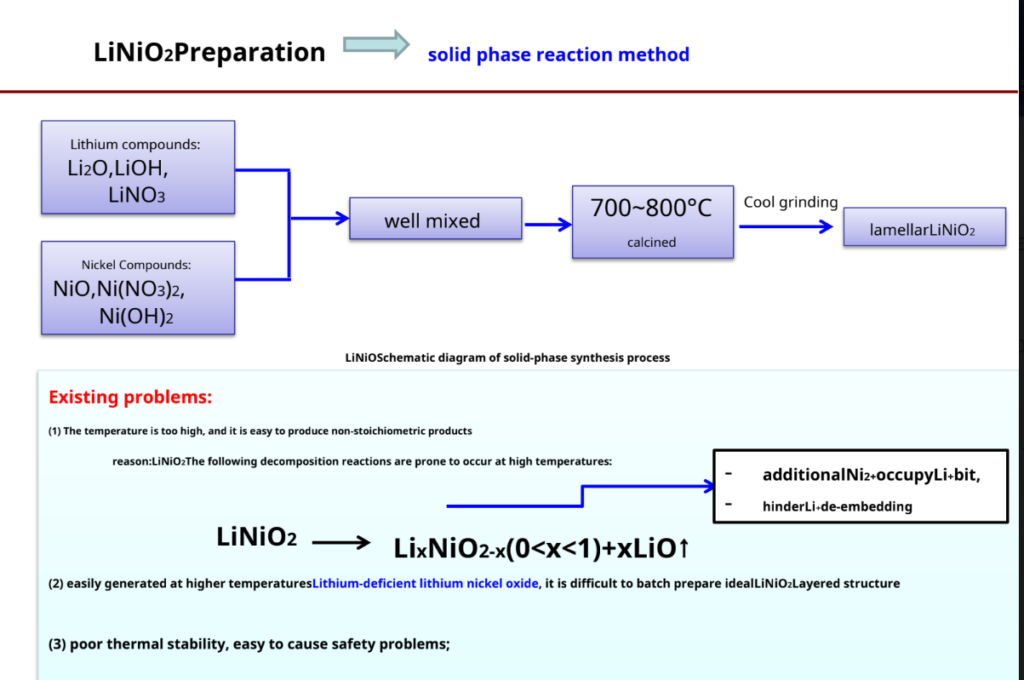
LiCoO2Preparation – most commonly used synthetic methods- solid phase reaction method
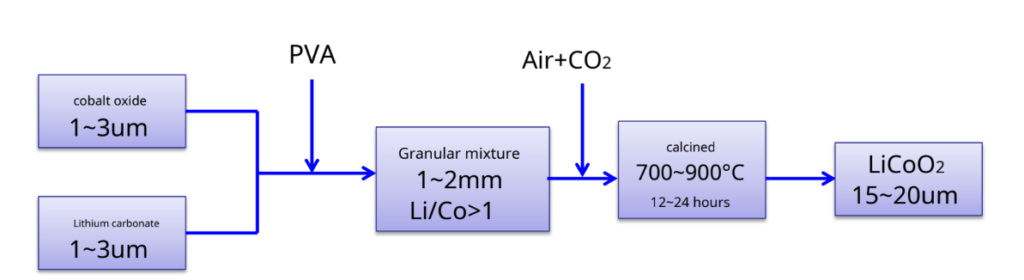
Produced by SonyLiCoO2Schematic diagram of
The process —–4Co3O4+ 6Li2CO3+ O2 12LiCoO2+ 6CO2↑
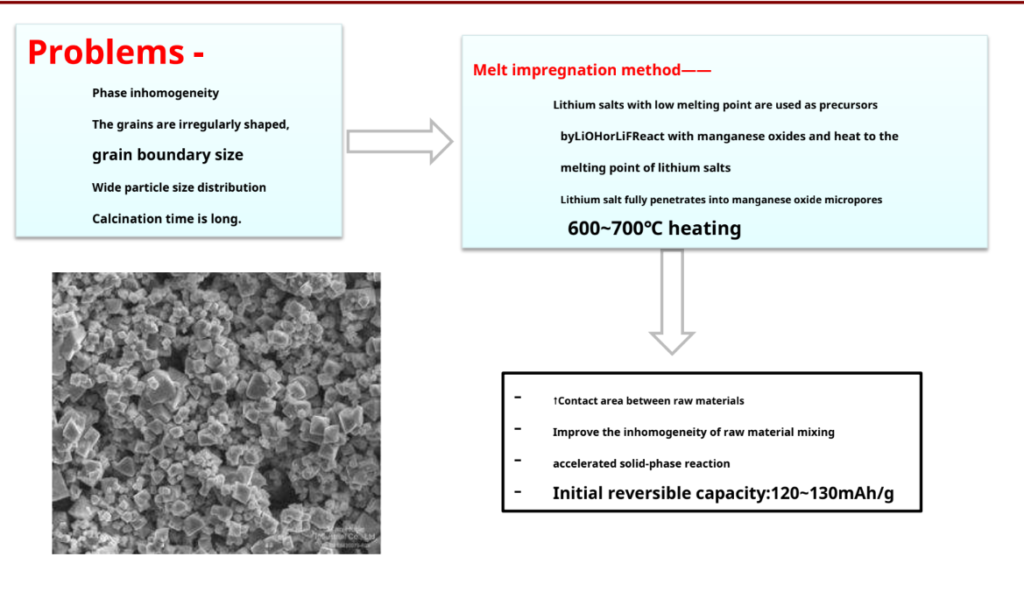
Advantage: Simple process, easy to operate, suitable for industrial production Insufficient: Materials are difficult to mix uniformly
High energy consumption
Larger particles and irregular shapes
Electrochemical performance reproducibility
LiCoO2Preparation – other synthetic methods
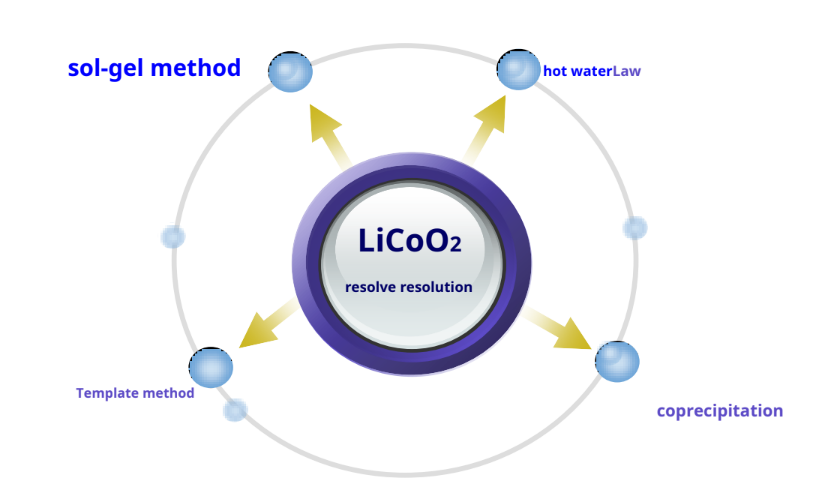
-Pros: Li+ and Co2+There is sufficient contact between them to achieve approximate atomic level mixing, and it is easy to control the particle size and phase composition of the product.
-Disadvantages: The process is cumbersome, the cost is high, and it is not easy to industrialize production.
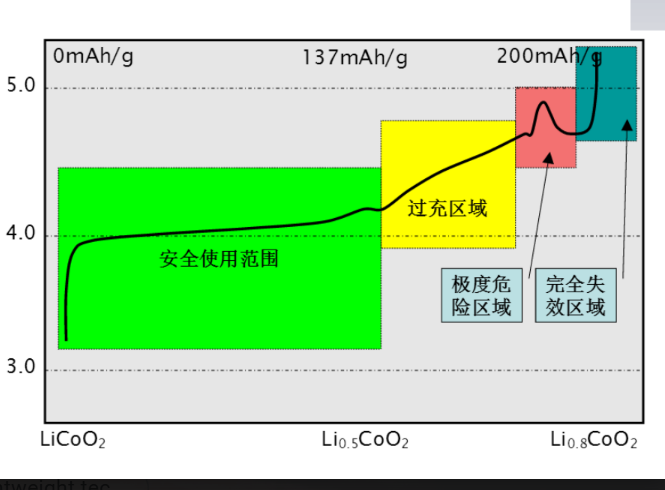
-The maximum proportion of reversible de
-intercalation: is about 55%
-Theoretical specific capacity: 274mAh/g-Actual specific capacity: 130-150 mAh /g
LiCoO2 specialty
The synthesis method is simple and easy to synthesize
High working voltage, stable charging and discharging voltage
High specific energy, suitable for high current charging and discharging
The actual capacity is lower, only the theoretical capacity50%
Cobalt resources are limited and expensive
Cobalt is highly toxic and pollutes the environment
Poor safety performance under charging conditions
Cycle performance needs to be improved
LiCoO2Study on Modification of Cathode Materials
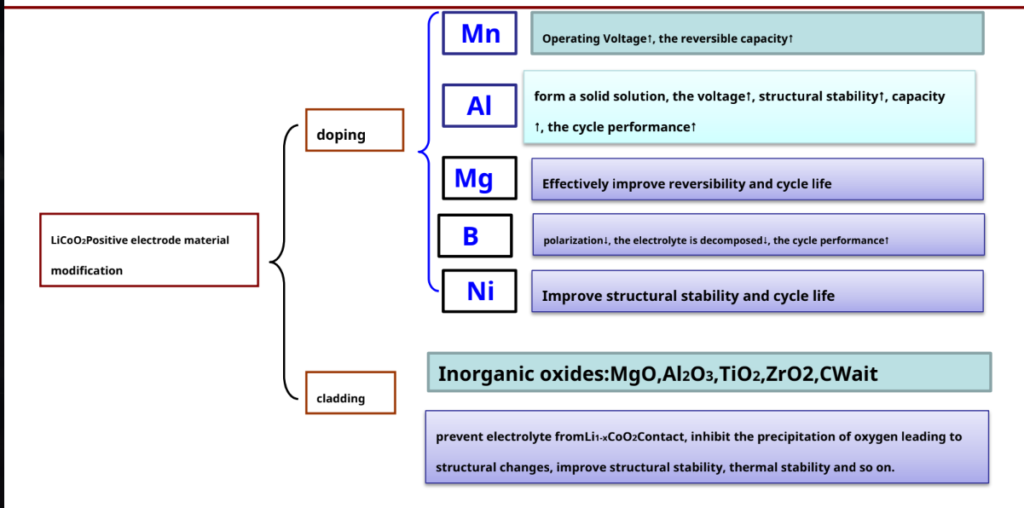
Future research directions – reduce LiCoO2 cost and increase at higher temperatures (<65°C) cycle performance.
Layered structure cathode material – LiNiO2Material
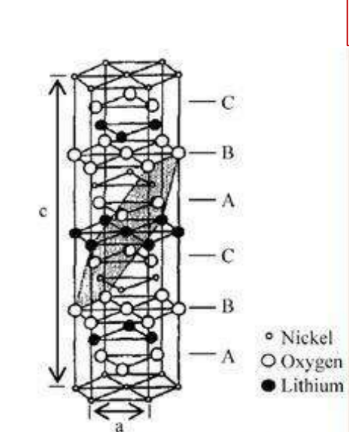
Sketch of crystal structure
Crystal structure: α-NaFeO2type layered structure, R3 m Space group
Lattice constant: a=0.2886nm
c=1.4214nm
☆ Oxygen atoms are located in position, is cubic close-packed, the nickel atoms are located in3aposition, the lithium atom is located in positions, alternately occupying octahedral positions
☆ exist [111] the crystal planes are arranged in layers Li+ Diffusion coefficient: 2×10-11m2/s
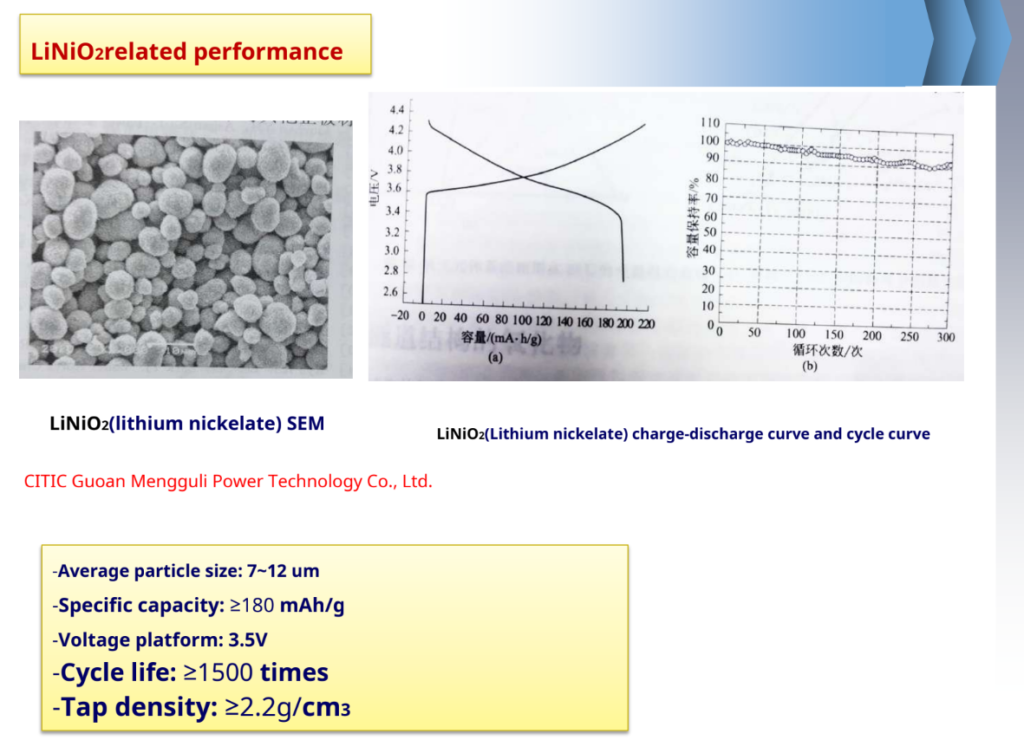
-Advantages: high capacity, the actual capacity is 190~210mAh/g; price and resource advantages, etc.
-Inadequate: poor thermal stability of the structure, high heat release.
Spinel structure cathode material – LiMn2O4Material
Spinel typeLiMn2O4belongFd3mspace group
The oxygen atoms are arranged in a cubic close-packed arrangement
- Occupies a face-centered cubic (32e) bit
- MN occupies an octahedron (16d) bit
- Li+ occupies a tetrahedron (8a) bit
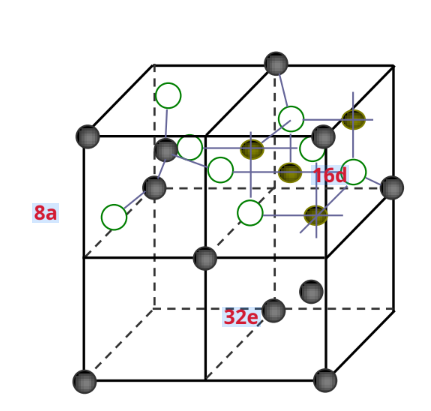
☆ Empty tetrahedral and octahedral are interconnected by co-planarity and co-edge, forming three-dimensional channels for lithium-ion diffusion.
-Diffusion coefficient: 10-14~10-12cm2/s
-Theoretical specific capacity: 148 mA h/g, the actual specific capacity is about 120mAh/g
Spinel LiMn2O4Conventional preparation of materials – solid-phase reaction method
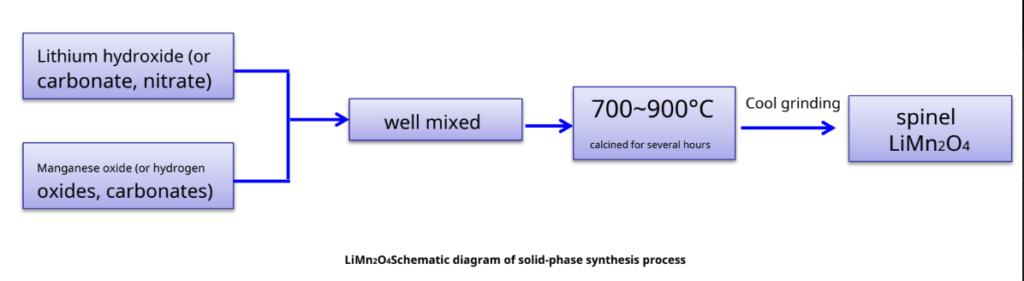
Taking lithium carbonate as an example, its specific reaction schematic diagram:
Li2CO3+2Mn2O3+1/2 O2 LiMn2O4+CO2
Li2CO3+2MnO2 LiMn2O4+1/2 O2
Spinel LiMn2O4Conventional preparation of materials – solid-phase reaction method
Spinel LiMn2O4Cause Analysis of Material Capacity Decay
LiMn2O4When the cathode material is cycled, especially at a high temperature (55°C), keeping its capacity decay problem, leading to poor cycle performance.
The reasons are analyzed as follows:
1 Dissolution of manganese- end of dischargeMn3+ the highest concentration is on the particle surfaceMn3+ a disproportionation reaction occurs:
2Mn3+ (solid) Mn4+ (solid) + Mn2+ (solution)
Produced by the disproportionation reactionMn2+dissolved in the electrolyte, on the one hand, Yes LiPF6 Formed by reaction with traces of water H Faced, accelerated MN the dissolution; therefore, MN the dissolution isLiMn2O4The main reason for the capacity loss.
Spinel LiMn2O4Cause Analysis of Material Capacity Decay
2. MN high oxidative
Highly delighted spinel Li in organic solvents1-xMn2O4 unstable at the end of charge, i.e. Mn4+of high oxidation.
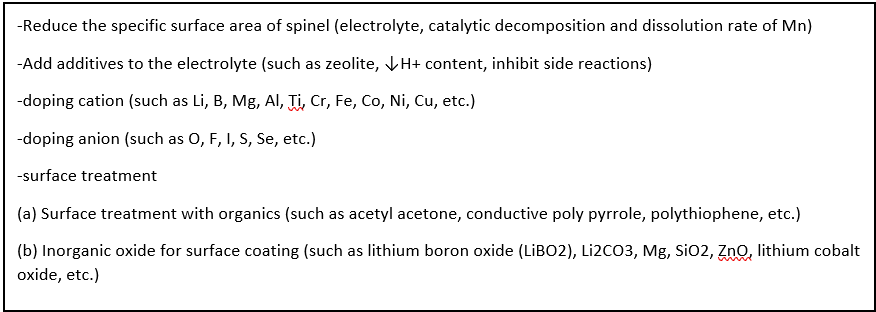
Spinel LiMn2O4Modification of materials
Theoretical specific capacity: 170 mAh/h
Crystal structure: PbnmOrthogonal space group
Lattice constant: a=0.6008nm
b=1.0334nm
c=0.4693nmUnit cell volume: 0.2914 nm3
The oxygen atoms are arranged in a slightly twisted hexagonal close-packed arrangement.
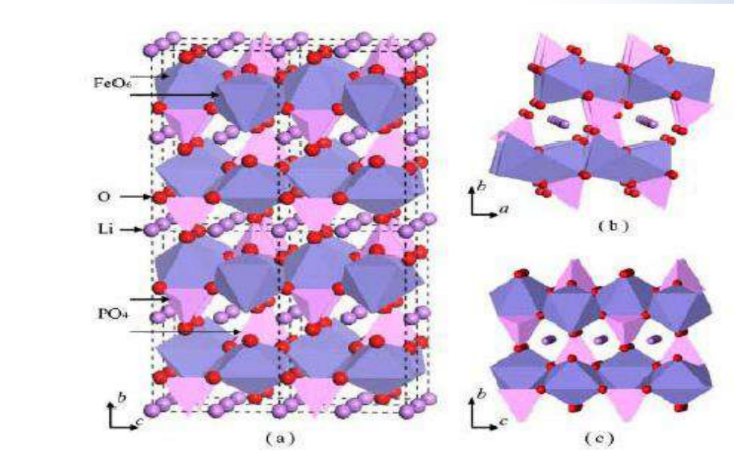
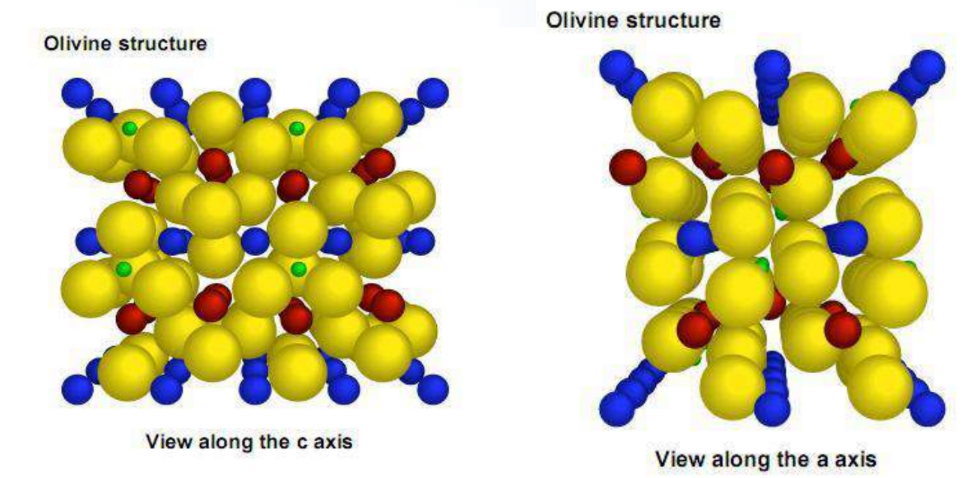
FeandLiare located at the center of the oxygen octahedron, respectively, formingFeO6andLiO6Octahedron.<br>Oxygen tetrahedra4cposition, formedPO4tetrahedron.<br>Li+two- dimensional diffusion motion.
During charging, lithium ions areFeO6It migrate out between the layers, enter the negative electrode through the electrolyte, and
occursFe2+→ Fe3+The oxidation reaction, in order to maintain charge balance, electrons from the external circuit to the negative electrode. During discharge, a reduction reaction occurs, which is the opposite of the above process. Which is:
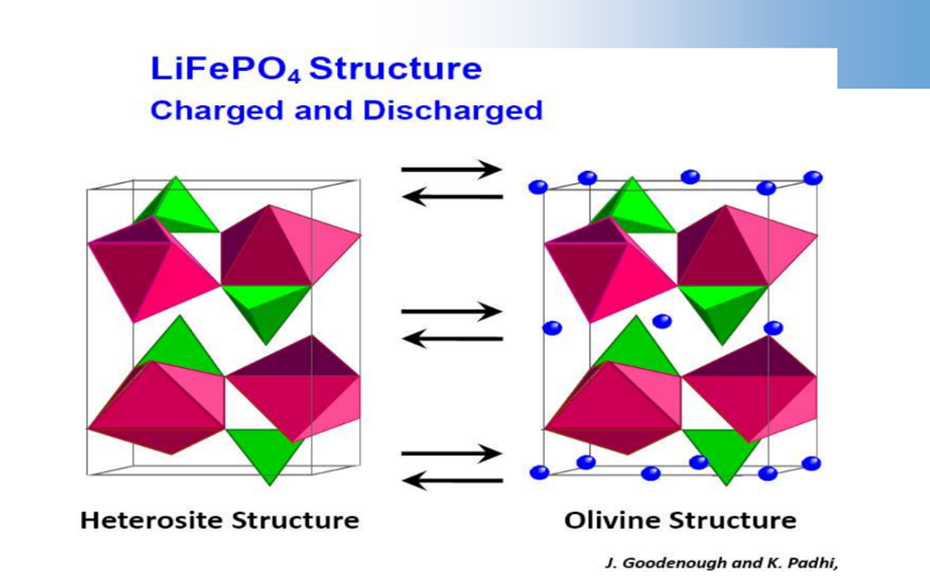
While charging: LiFePO4-xLi+-xe-→xFePO4 + (1-x) LiFePO4
When discharging: FePO4+xLi++xe-→xLiFePO4+ (1-x) FePO4
Olivine structure cathode material – LiFePO4
LiFePO4structure determines it’s only suitable for charging and discharging at low current density.
LiFePO4The delithiation product ofFePO4, the actual charging, and discharging process is in FePO4/LiFePO4two-phase coexistence.
FePO4andLiFePO4The structure is very similar, the volume is also close, and the difference is6.81%.
Good cycle performance!
Lithium iron phosphate synthesis method
Since 20century 90 the late 1990s, olivine-type lithium iron phosphate (LiFePO4) research on cathode materials has attracted the attention of many researchers. It is expected to become the preferred alternative for the new generationLiCoO2Lithium-ion battery cathode material, especially as a powerful lithium-ion battery cathode material.
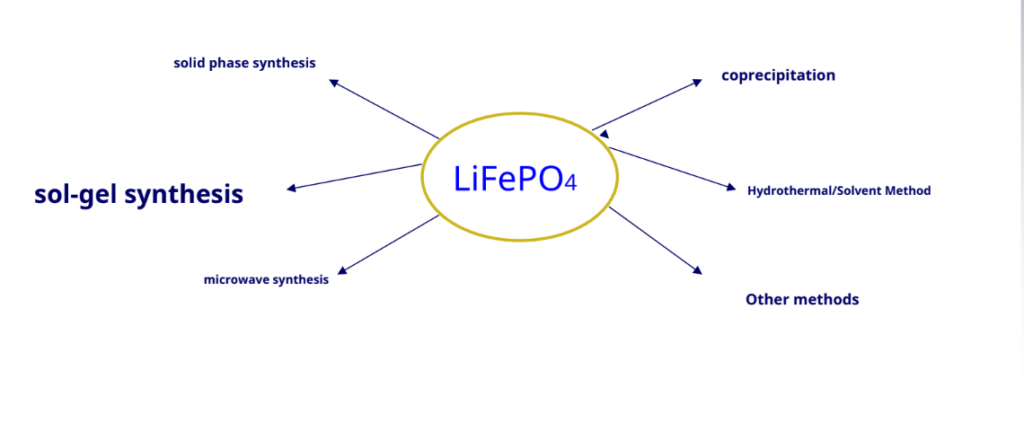
Solid phase synthesis: –
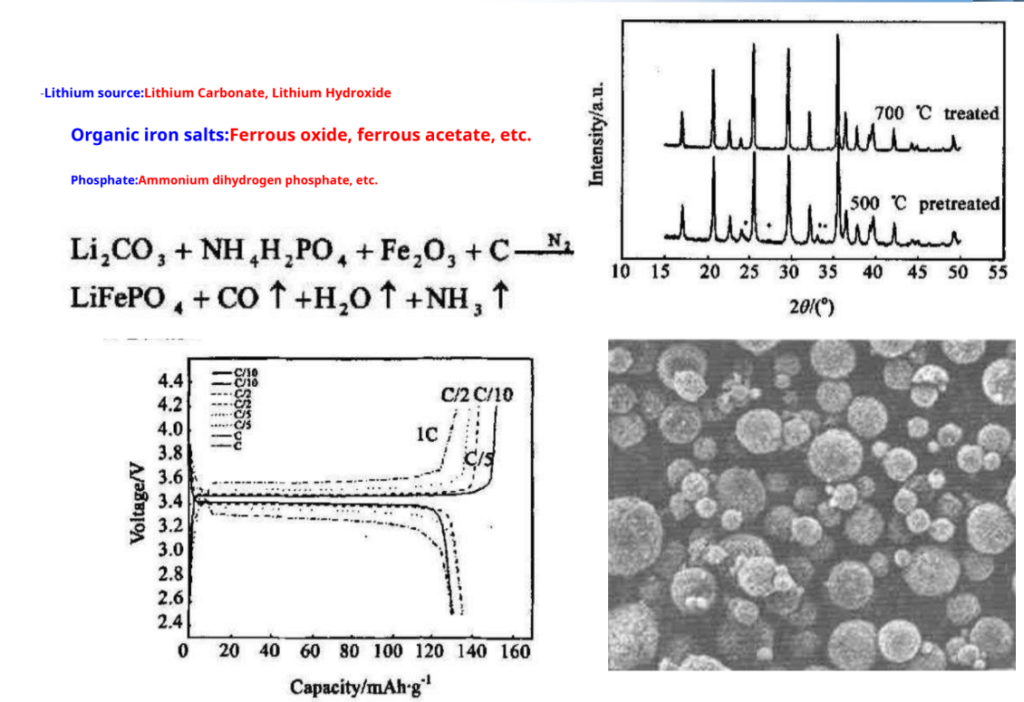
Hydrothermal synthesis
-Hydrothermal synthesis means that the temperature is100~1000°C, and the pressure is1MPa~1GPa used under conditions chemical reactions of substances in water the synthesis performed.
-Under subcritical and supercritical hydrothermal conditions, due to the reaction being at the molecular level and the reactivity increases therefore, hydrothermal reactions can replace some high-temperature solid-phase reactions.

Compared with the high-temperature solid phase method, the use of the hydrothermal method LiFePO4.It has the advantages of high product purity, uniform phase, good dispensability, small particle size, and easy operation.
LiFePO4Although having the structure is stable, safe, pollution-free and cheap Etc. presence of lithium ions small diffusion coefficient, low electronic conductivity, etc. disadvantages, resulting in its cycle performance at room temperature and High charge and discharge performance Not very good.
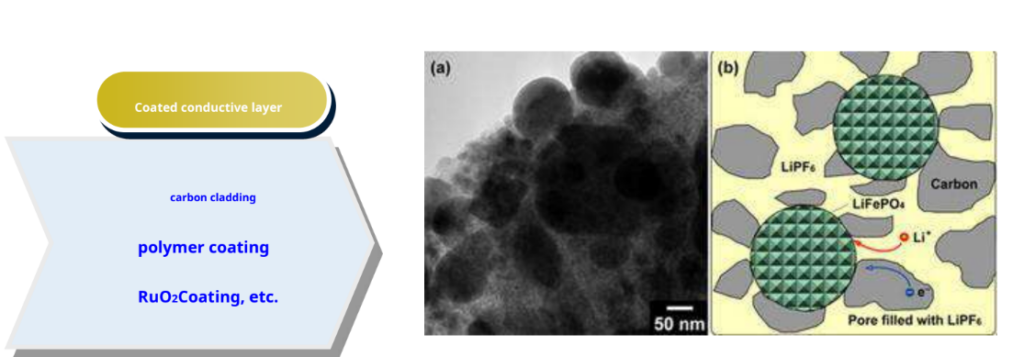
-The method of adding carbon to synthetic precursors was first proposed by Ravet et al. [1] of the Goodenough group. It has three functions:
- As a reducing agent, avoid the formation of the trivalent phase at lower temperatures;
- Prevent the contact between particles and refine the grains;
- Enhance the electronic conductance inside and between particles.
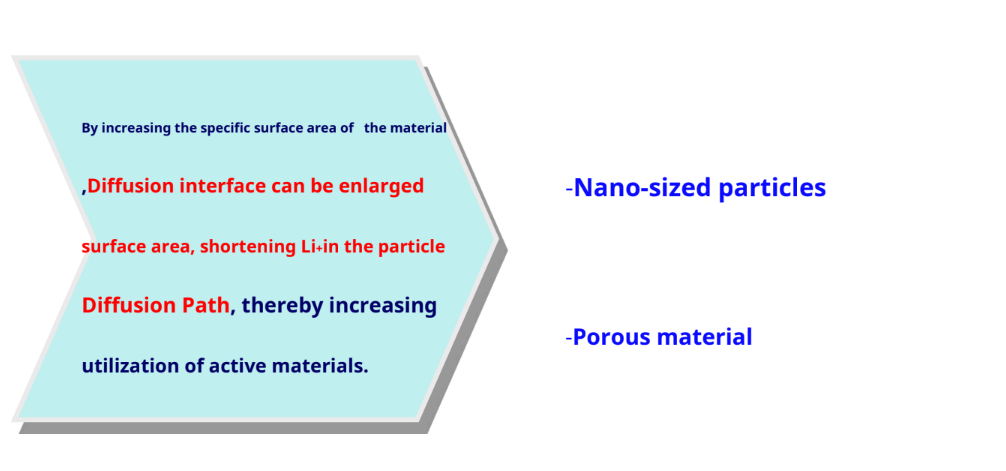
Ion doping

Increase specific surface area



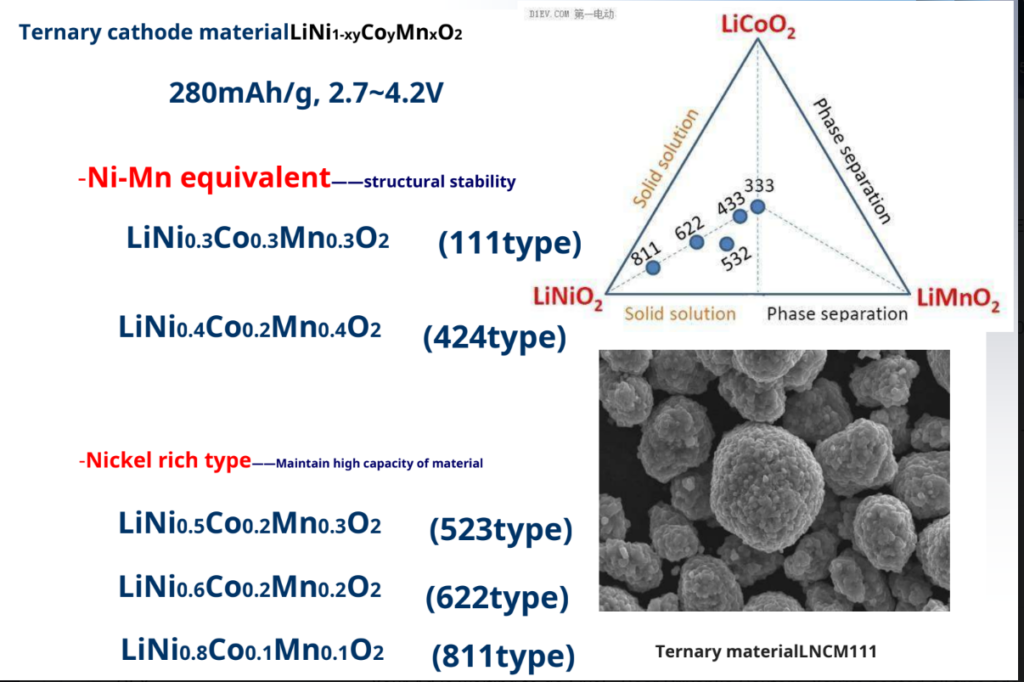
Ternary cathode material – Li (Ni, Co, Mn)O2
Ni-Co-MN Synergy – LiCoO2good cycle performance
– LiNiO2High specific capacity
– LiMnO2High security and low cost
Crystal structure: α-NaFeO2type layered structure, hexagonal system, and R3m space group.
☆ Li+ alternating with transition metal ions3abit (000) and 3b (0 0 1/2) bits, O2-lie in6cbit
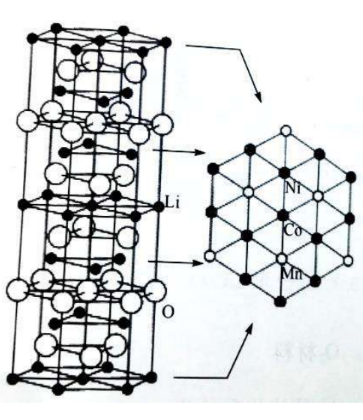
Schematic diagram of the layered structure of lithium manganese cobalt oxide ternary material
Ternary cathode material
The manganese sulfate crystals, nickel sulfate crystals, and cobalt sulfate crystals are prepared in different proportions and added Na OH solution, ammonia reaction generation Nickel Cobalt Manganese Hydroxide Precursor, then add Lithium source (lithium carbonate)Mix and stir at high speed, then proceed High-temperature sintering, crushing and sieving to remove iron, and finally form a ternary material precursor.
Sol-gel method
Principle: This method is a relatively advanced soft chemical method for synthesizing ultrafine particles. It is mainly used in the synthesis of various ceramic powders, coatings, films, fibers, and other products. Prepared by this method NCM First, the low-viscosity precursors are mixed uniformly to make a uniform sol, then gelatinized, and then obtained by molding, drying, sintering/calcining NCM Material.
Advantages: The biggest advantage is that the reactants can be uniformly mixed at the molecular level in a very short time, and the prepared materials have chemicals.
Uniform distribution of components, accurate stoichiometric ratio, small particle size, and narrow distribution. The latest research found that the use of ethylene glycol as a dispersant can synthesize uniform particle size and carbon-coated carbon in one step.333Ternary materials, the capacity retention rate is still high after high-rate discharge, in addition, the prepared by this method424Type materials have higher discharge capacity.
Disadvantages: the preparation cost is high, the process is complicated, the method has large environmental pollution, and the industrial production is difficult.
Solid phase method
Founder of Ternary Materials OHZUKU Originally synthesized by solid-phase method333 Materials, traditional solid-phase methods simply use mechanical mixing.
Therefore, it is difficult to obtain ternary materials with uniform particle sizes and stable electrochemical performance. At present, the primary particle size of the material obtained by the improved solid-phase method is in the range of 100-500nmHowever, due to high-temperature sintering, the primary nanoparticles are very easy to agglomerate into secondary particles of different sizes, so the method itself needs to be further improved.
Other preparation methods
-Template method: Using carbon fiber as a templating agent, the carboxyl group (-COOH) on the surface of carbon fiber is used to adsorb metal nickel, cobalt, and manganese ions, and the Nanoporous 333 ternary material can be obtained after high-temperature roasting.
By virtue of spatial confinement and orientation, the template method is widely used in the preparation of materials with special morphologies and precise particle sizes. On the one hand, the Nanoporous 333 ternary material prepared by this method can greatly shorten the diffusion path of lithium ions, and on the other hand, the Nanoporous structure can buffer the volume change of the material, thereby improving the stability of the material.
-Spray drying method: At a high temperature of 60-150 °C, the nickel cobalt manganese lithium nitrate is rapidly atomized, the water evaporates in a short time, and the raw materials are quickly mixed. . The spray drying method is regarded as a method for producing ternary materials with very broad application prospects due to its advantages of the high degree of automation, short preparation period, fine particles obtained, narrow particle size distribution, and no industrial wastewater.
Conclusion
Lithium-Ion batteries are a great power source for projects but they require care during use and charging. They can be easy to damage or misuse and can hurt also! All batteries should be tested. The following points must be followed during the manufacturing process:
- Do NOT immerse the battery in water or other liquids. Keep or store the battery in a cool and dry place/environment.
- Do NOT use or store the battery near any source of heat.
- Use a charger that is clearly specified to be compatible with charging the battery and has appropriate charging protection (voltage, current, temperature)
- Do NOT install the battery in reverse polarity.
- Do NOT connect the battery to an electrical outlet or other incompatible power sources.
- Do NOT discard the battery in fire.
More Articles:
Basic knowledge-manufacturing process of lithium,
The packaging form of lithium-ion battery is better,
Causes Reconditioning Of Battery Vulcanization,
Lithium-ion Battery Process Details,
Lithium battery overcharge mechanism and anti-overcharge measures.,
Factors Affecting The Performance of High Power Lithium-ion Batteries,
Electrical measurement of lithium-ion batteries,
Functions of the Battery management system (BMS),
Why do electric vehicles get slower and slower after riding,
why can’t Electric Cars of the same model overt beat,
what to do if the electric car is flooded,


