Introduction
During the first cycle of the battery, there are processes such as the formation of negative electrode sei, the deactivation of negative electrode material particles due to falling off, and the irreversible deposition of lithium metal, which consumes the energy of the positive electrode. Active lithium, reducing the available energy of the battery. Lithium supplementation technology (Pre Lithiation Strategies for Lithium Battery) is used to pre-compensate for the loss of active lithium during the first cycle of charge and discharge.
- Increase the reversible capacity of the battery in the first week, increase actual battery energy density.
- Realize the pre-expansion of the negative electrode material volume, reduce the cracking and polarization of material particles during the lithium intercalation process, and improve the mechanical stability and cyclability of the negative electrode
- Some lithium-replenishment techniques can form artificial sei films, which can replace the chemical formation step of the battery.

Definition: By increasing the content of the active lithium source, pre-compensation loss of active lithium during the first week of charge and discharge
Factors to consider for pre-lithiation:
- The degree of pre-lithiation, over-lithiation will cause Li dendrites, matching with cathode materials (especially those without Li source)
- In addition to Li, are there any other inactive residual substances in the decomposition products of the pre lithiation reagent? thereby reducing the battery.
- Pre-lithiation time, too long will increase production cost
- Compatibility between pre-lithiation process and existing battery production process
- The price of lithiation reagent

Buy Now: SEMCO Lithium Battery Charge & Discharge Unit SI CDM 100V 30A
Negative pre-lithiation (Chemical Method)
Lithium Sheet
The negative electrode is in direct contact with the lithium foil. In the electrolyte environment, the lithium foil is in contact with the negative material potential difference leading to a directional movement of the electron flow, the li generated in the lithium foil + released. In the electrolyte, in order to maintain the charge balance, the li in the electrolyte + insert/embedded in the negative electrode material.

Inert Lithium Powder (FMC Lithium Corp.)
Lithium powder is unstable in air; therefore, a layer of Li is coated on its surface 2CO3, significantly improving its performance in dry air stability. Activation process: pressure is applied to inert lithium powder, Li2CO3The layer is broken, so that the inner Li powder and the outer. The electrode active material and electrolyte come into contact with each other.

Method 1: Spread inert lithium powder particles on the surface of the electrode
Method 2: Disperse the inert lithium powder particles in a non-polar solvent to form a suspension, and spray evenly on the surface of the electrode with a spray gun
Buy Now: SEMCO Battery Charge & Discharge Cabinet Tester SI BCDS 99V – 20A – 1CH.
Insert Lithium Powder
Method 3: Mix inert lithium powder into the electrode slurry.


Summary
| Lithium foil | Lithium powder | |
| Specification | Thickness≈100 -200μm | Particles ≈ 10-100 μm, the surface is usually |
| Advantage | Cheap | The degree of lithiation is controllableLithium powder can be completely dissolved in the electrode, no need takes out. |
| Disadvantages | The degree of lithiation is not easy to controlLithium foil needs to be removed before battery assembly, unlike existing Commercial production process is not compatible | ExpensiveLight density and poor dispersion in electrodesChemically unstable, it needs to be used in non-polar solvents and use in dry air |
| Dynamics | The surface of the lithium foil has a microporous structure and the thinner the thicknessHelps electrolyte infiltration and shortens lithiation time | Faster lithiation kinetics |
Li-organic Complex Solution

Due to the strong electron affinity of naphthalene, lithium metal can be dissolved in ether-based solvents, forming into [Li+-Naphthalene-] complex solution with lower reduction potential for lithiation negative electrode material. Principle: The electrons of naphthalene are transferred to the negative electrode material, as compensation, Li+ into the negative electrode material. Naphthalene as Electron Transfer Catalyst.

- Naphthalene can be replaced by biphenyl etc.
- Methyl butyl ether can be replaced with other ether-based solvents such as THF, and the solvent has a great influence on the lithiation rate
- SiO can be replaced with LTO, SnO, P, etc.
Lithium Anode Material
The negative electrode material is reacted with molten lithium or ball-milled with lithium to obtain a lithiated negative electrode material. Then coat the surface, Improve its stability in dry air. Add it to the negative electrode slurry to lithiated the negative electrode.

Highly Reactive with Air

Lix Advantages of Si nanoparticles:
- The potential is very low, and almost all anode materials can be lithiated
- High lithiation efficiency (4200 mAh g for Si-1, Li4.4Si is 2000 mAh g-1)
- Nanoscale size facilitates uniform dispersion in anode, accelerating lithiation kinetics
- No need to change the original battery production process, there is the potential for mass production.
Buy Now: SEMCO provide high-quality Battery Balancer to measure the voltage, resistance, and more.
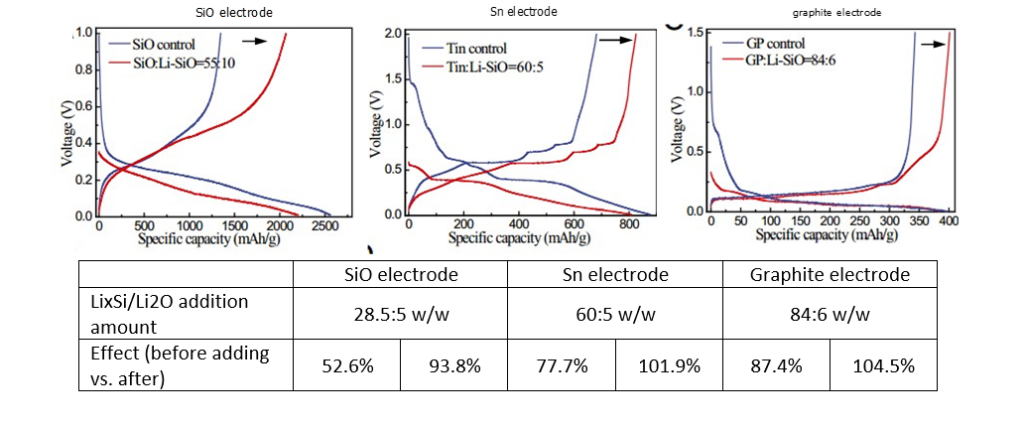
In slurries in different solvents, LixSi-Li2First Week DE lithiation of O

LixSi to carbonyl carbon nucleophilic attack strong ability

- LixSi is not compatible with polar solvents such as NMP and DEC
- LixSi is compatible with non-polar solvents such as DOL ethers and toluene
- PVDF can only be dissolved in DOL, so DOL is used as the solvent for the slurry
Li2O Coated LixSi

- LixSi-Li2The pre-lithiation capacity of O is 1310 mAh g-1,
- LixSi-Li2The lithium storage capacity of O is only 1710mAh g-1(LixSi-Li2O<Li4.4Si < Si), Description LixA large part of the capacity of Si irreversibly consumed to form Li2O passivation layer.

Artificial SEI Coated LixSi

- Than LixSi-Li2O, artificial SEI-coated LixSi has higher stability and stronger lithiation ability.
- Fluorinated decane molecules have a long-chain structure like surfactants, so they can be dissolved in non-polar solvents (LixSi can also exist stably), and in Lix Reduction and decomposition of Si surface to form artificial SEI protective film.
- Adjust the concentration of fluorinated decane solution and adjust the thickness of SEI film.

- Lix one electron of SI is transferred to CF of 1-fluorodecane, forming C radical and F-, the second electron makes C free radicals are converted into C negative ions.
- Alkyl lithium is extremely volatile and can spontaneously interact with trace O in the glove box.2and CO2 react, produce Lithium Decyl Carbonate.

- OCV drops to 0.27 V, indicating lithiation
- Voltage plateau at 0.4 V, demonstrating that Lix Crystal Properties of Si
- Li after coating with artificial SEIxSi capacity reduction10%, which proves that approximately 10% of active lithium is consumed to form SEI. However, the package LixSi is only used as anode additive, so its capacity loss is negligible.


Chemical Stability Test

- LixSi is less stable in dry air
- The artificial SEI-coated LixSi has better stability in dry air, and the capacity decays only negligibly after 5 days.
- Exist 10% Under humidity (similar to the industrial production environment of batteries), the coated LixSi still has higher capacity.

- LixSi-LixNySiz10th charging capacity of 2808mAh g-1
- With N2Passivated LixSi surface, coating LixNySiz The higher the N content, the higher the Lix The higher the stability of Si,
- LixNySizSi is electrochemically inert, and the higher the Si content, the more LixSi-LixNySiz the lower the capacity.

Chemical Stability Test

Crystallinity Significantly affects the stability of the material. The higher the crystallinity of the coating matrix or coating, the better the chemical stability. Exist high temperature, the high crystallinity Li formed 2O substrate, with the normal temperature Amorphous Li formed under2O compared to (core shell Li in the structure2O), denser, can prevent side reactions with air.

Chemical Stability Test
Molten Lithium

Lix The stability of Si/graphene embedded structure is better than that of LixSi/Li2O embedded junction structure, thanks to the hydrophobicity and gas impermeability of the surface graphene layer Lix Electrochemical performance of Si/graphene anodes.

- Lix The Si/graphene composite was used as the anode material, and the lithium-containing cathode (LiFePO4) and Li-free cathodes (high-capacity S and V2O5) to match the full battery,
- The first-week efficiency, cycle stability, and rate performance were improved, respectively, thanks to the pre-lithiation of the negative electrode; the negative electrode material Pre-expansion of material volume; ultra-high conductivity of graphene.
Summary

Li-OH at High Temperature


- ¼ of SiO transforms into Li4SiO4, library 11% increase in efficiency,
- Increased capacity retention,
- Li4SiO4 electrochemically inactive, Can Inverse capacity reduction.
Thermally Evaporated Lithium

There are three types of products:
- Reversible Li
- Non-dischargeable Li, Li-Si alloys
- Irreversible Lithium in the Inactive Phase
Li was placed in a Ta boat, heated under vacuum, and Li evaporation was controlled by measuring Li deposited on Cu can better control the degree of lithiation.
Anode Pre-lithiation – Summary of Chemical Methods
- Control the degree of lithiation by adjusting the reaction time or the concentration of the lithiation reagent,
- Lithium reagents have high reactivity, and some even have potential safety hazards. The reaction conditions are harsh (dry air, non-polar solvents),
- Compatibility with commercial production processes needs to be improved,
Negative Pre-lithiation (Electrochemical Method)
Anode Material and Li-Sheet Assembled Battery:

- After lithiation is achieved, it involves battery disassembly and reassembly,
- Electrolyte volatilization problem,
- Cathode Additives,
- Over lithiated cathode material,
Cathode Additives

During the first week of charging, the excess Li in the positive electrode additive will migrate to the negative electrode, compensating for the loss of active lithium in the negative electrode.

- The irreversible lithium release process of the additive should be within the working potential range of the positive electrode,
- The additive should have a high specific capacity to ensure efficient pre-lithiation,
- Additives should be compatible with NMP, air and existing battery production processes,
- The chemical, electrochemical, thermal and mechanical stability of the product after the lithium release of the additive is good 37.

- Li3N capacity ≈ 1400 mAh g-1,
- Li3N oxidative decomposition without residue: Li3N → N2+ Li,
- Li3N ions are conductive but electronically insulating. When dispersed as an additive inside the electrode, it affects the rate performance,
- Li3N coating on the electrode surface has no effect on the rate performance,
- Li3N is stable in dry air and reacts with polar solvents (Li3N + H2O → Li-OH + NH3),
- Li3The N particles are too large to exert electrochemical activity and need to be ground.

Li2S/Co Complex
- The complex can fix the intermediate polysulfide and prevent it from irreversibly reacting with the carbonate electrolyte,
- The composite exhibits good air stability.

- Li2The charge capacity of the S/Co composite is 711 mAh g-1, the discharge capacity above 2.5 V is only 13 mAh g-1,
- Charging response: Co + 2Li2S → CoS2+ 4Li++4e-
- The lithiation voltage is at 2.5 V, within the working voltage of the cathode material, and the release of lithium is an irreversible process,

- Chemical preparation: CoF3+ 3Li → Co + 3LiF,
- Co nanoparticles are uniformly embedded in the LiF matrix,

- The transformation mechanism releases lithium: Co + 3LiF → CoF3+ 3Li++ 3e-,
- Lithium release capacity 516 mAh g-1,

- Chemical preparation of Li2O/Co: Co3O4+ 8Li → 3Co + 4Li2O,
- When the Co particles are larger, the composites exhibit higher charging voltage and less capacity, indicating that the larger Co particles will lead to Li2Poor contact between O and Co particles,
- Nanoscale Li2O/Co capacity of 619 mAh g-1, conversion reaction: 3Co + 4Li2O → Co3O4+ 8Li++ 8e-, but Co3O4Residues not only add weight, but also cause side reactions,
- Li2O2→ Li + O2
- Transition metals Ni, Co and Mn in NCM can catalyze Li2O2oxidative decomposition,
- NMC particles need to be ball milled to a smaller particle size to help Li2O2decomposition, however the electrochemical performance of smaller particle size NMC is poor, reversibility is poor, and inactive residues are produced.

- Li2NiO2The first week of lithium insertion capacity is 340mAh g-1, de-lithiation capacity 83mAh g-1
- After the second week, Li2NiO2Delithiation capacity 76 mAh g-1, and thus can also partially act as an active material
- Li2NiO2Instability in air, using Al2O3cladding
- wt.%Li2NiO2can compensate LiCoO2First week irreversible capacity loss for graphite

- Li5FeO4The theoretical capacity of 867 mAh g-1,
- 7wt% Li5FeO4can compensate LiCoO2/Hard carbon first week irreversible capacity loss,
- Li2NiO2with air moisture and CO2 reaction,


- Lithium salts all have 300-600 mAh g-1ofTheoretical capacity, working voltage is 3 – 4V
- Oxidative decomposition without residue, generating Li and gas N2, CO2
Has not yet been applied to batteries?
Buy Now: Semco Infratech Cell Testing Machine CT 5V – (5A-100A) – (8CH-512CH)
Summary of Cathode Additives

- In terms of capacity, binary additives are generally larger than ternary additives, except for Li5FeO4 outside.
- Focus on the residues after decomposition, especially inactive substances.


Lithium over-intercalated cathode material with LiNi0.5Mn1.5O4For example, the 4.7V platform corresponds to its Tetrahedral site Lithium extraction/intercalation. 2.7V will further, Lithium intercalation, Li+ will embed 16c octahedral site, form o-LNMO. But the lithium intercalation at this low potential is irreversible. Therefore, low the voltage cycling is only used for the first cycle, the released Li can be supplied to the negative electrode, and then the standard potential interval cycle is resumed.


More Articles:
Latest Technology of Lithium Battery,
Dry Goods Lithium Button Battery Assembly and Test,
MSDS For LiFePO4 Battery Pack,
Power Battery Pack Technology,
Best Way of Charging Lithium Batteries,
Analyze Common Bulging Reasons for Lithium Batteries,
Aging of NCA Lithium Batteries,
Function and Components of Battery Pack & BMS,
Cell BMS Pack Failure Analysis,
Lithium Battery Protection Board,
Basic Knowledge of Lithium-ion Battery Commercialization,


