Lithium-ion power battery is a complex system composed of positive and negative electrodes, positive and negative electrodes, electrolyte and diaphragm. The only thing that should happen is the implantation and release of ions between the electrolytes and positive and negative electrodes. There are no other adverse effects observed. The reaction does not result in the loss of lithium ions, nor will the battery’s capacity.
Nevertheless, in real life, side effects will happen inside the battery in addition to lithium ion embedding and ejection. Lithium-ion loss, electrolyte loss, active substance consumption, and ultimately attenuation of battery capacity are all consequences of the side reaction. There are two main categories of causes for battery capacity attenuation: internal and external.
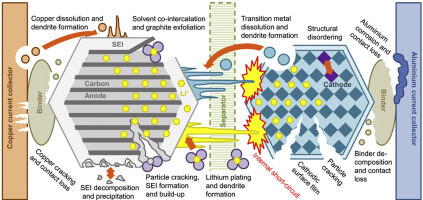
The internal reasons for lithium battery capacity attenuation mainly include electrode changes, electrolyte loss, SEI film, and diaphragm aging.
- Electrode change: Battery capacity will fluctuate throughout the battery cycle due to the loss of active materials, the change of positive materials, and the change in collector fluidity. The electrode material will break down as a result of adverse reactions. The expansion of the positive material structure brought on by the embedding and release of lithium ions will also affect the battery capacity by preventing the de-embedding of lithium ions and causing a phase change in the positive material structure.
- Electrolyte loss: During the battery cycle, the side reaction in the battery will consume the electrolyte material and affect the battery capacity.
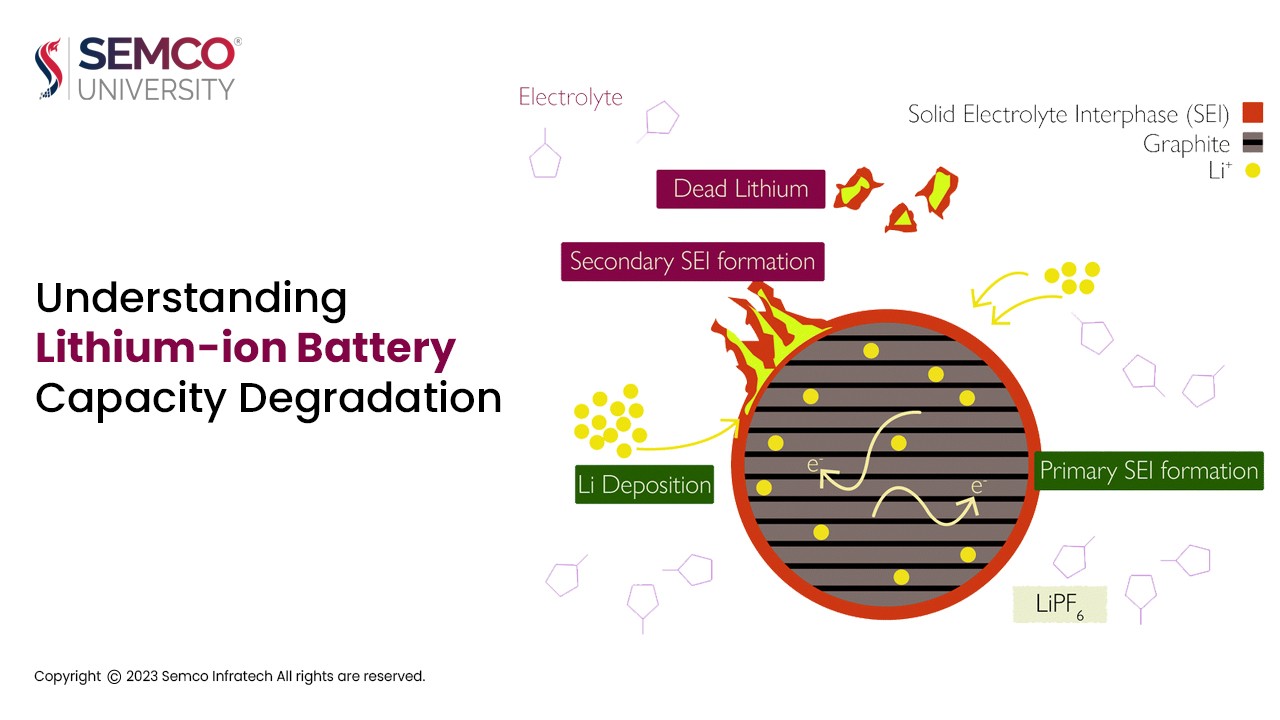
- SEI film: A solid electrolyte film (SEI film) is created at the start of the battery’s life by a reaction between the electrolyte and the electrode. The SEI film’s chemical properties are stable, and subsequent reactions will be coordinated following the formation of the electrode-electrolyte interface. Although lithium ions can be embedded and released on the electrode through the SEI membrane, stress will cause the membrane to burst, resulting in the rapid generation of a new SEI film. The loss of electrolyte and lithium ions resulting from the formation, rupture, and repair of the SEI membrane will ultimately cause the battery’s capacity to diminish.
- Diaphragm aging: The diaphragm’s roles include keeping the battery’s positive and negative electrodes apart and guarding against internal short circuits. The diaphragm should ideally remain unchanged. But the electrolyte will break down due to the battery side reaction, which will impact the diaphragm’s mechanical and electrochemical characteristics. This will raise the battery’s ohm resistance, which will reduce its capacity and shorten its lifespan.
The production of gas, ambient temperature, deep charge and discharge of the battery, and battery self-discharge are the primary external causes of lithium battery capacity attenuation.
- Battery self-discharge and deep charge and discharge: A retained battery will gradually discharge on its own. A chemical process called self-discharge takes place within the battery. Lithium ions and electrolytes will be consumed in this reaction, which will also produce substances that are insoluble in electrolyte and adhere to its surface, thereby influencing the embedding and release of lithium ions. Similar to deep discharge, overcharging, and deep discharge, excess lithium ions will also be produced by the positive and negative electrodes. The electrolyte and lithium ions react, and the resultant product influences the release and embedding of lithium ions. Both will ultimately result in a lower capacity and shorter lifespan for the battery.
- Ambient temperature: Battery capacity will decrease at low temperatures due to the electrolyte’s rapid decline in conductivity. The materials that make up the electrolyte and electrode will break down at high temperatures, which will immediately lower the battery’s capacity.
- Gas generation: The battery’s internal air pressure may become uneven, and its capacity may decrease as a result of side reactions that occur during battery use and produce other liquids or gases.
The battery capacity can also be impacted by additional factors. For instance, poor battery design, issues with the manufacturing process, and incorrect use will result in a reduction in battery capacity. Unreasonable design, for instance, could result in an unstable internal structure or other battery defects; issues with the manufacturing process could cause an uneven distribution of electrode materials or electrolyte leakage; and improper use could expose the battery to unfavorable conditions like over-discharge, overcharging, or high temperatures.
In summary, a variety of factors work together to reduce the capacity of lithium-powered batteries. We must be mindful of the use conditions, maintenance, and proper usage of the battery in order to preserve its capacity and lifespan. Simultaneously, it is anticipated that as science and technology continue to advance, more sophisticated battery technology and maintenance techniques will be developed to extend battery life and enhance performance.
For More Updates Follow Us
WhatsApp – Facebook – Instagram – Twitter – LinkedIn – YouTube

